Am Fam Physician. 2019;100(6):329-330
Published online July 29, 2019.
Author disclosure: No relevant financial affiliations.
Measles is one of the most highly transmissible diseases, with 90% of unvaccinated persons infected after contact.1 In 2000, the Centers for Disease Control and Prevention (CDC) declared that measles was eliminated in the United States, but that elimination status may be revoked if the current chain of measles transmission and outbreaks continues for one year, through the fall of 2019. As of July 18, 2019, a total of 1,148 measles cases have been confirmed in 30 states.2 Some patients underestimate the severity of measles, fear vaccine adverse effects, and refuse vaccination. Nonmedical vaccine exemptions are on the rise.3 The CDC estimates that about one in five unvaccinated people in the United States who contract measles will be hospitalized, one in 1,000 people with measles will develop encephalitis, and nearly one to three in 1,000 children with measles will die from respiratory or neurologic complications.4
Family physicians can help prevent the spread of measles by identifying and reporting suspected measles cases, following the Advisory Committee on Immunization Practices' measles-mumps-rubella (MMR) vaccine recommendations, assessing patient immunity status, addressing misconceptions about the MMR vaccine, and educating patients and legislators to reduce nonmedical vaccine exemptions.
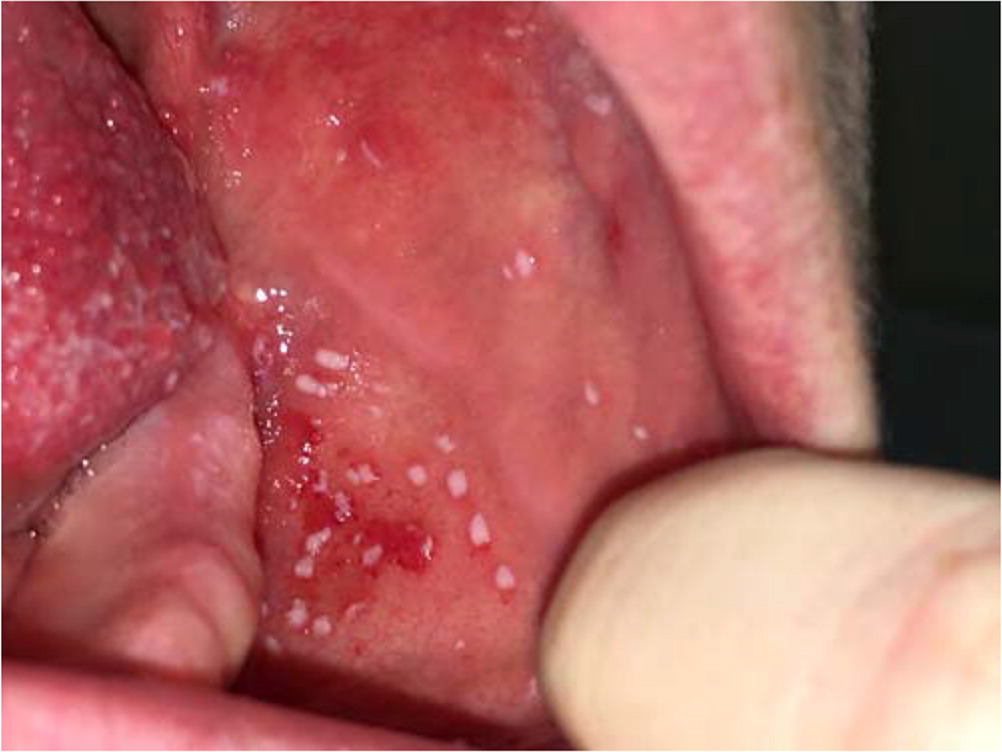
Measles typically presents seven to 14 days after exposure with a prodrome of high fever, malaise, cough, coryza, conjunctivitis, and a pathognomonic enanthem (Koplik spots) on the oral mucosa (Figure 15) followed by a maculopapular rash.1 The rash usually appears 14 days after exposure or three to five days after the first symptoms. It begins on the face at the hairline and spreads downward; it may not develop in patients who are immunocompromised. Patients are considered contagious from four days before to four days after the rash appears.1
Clinicians are obligated to report suspected cases to their local health departments within 24 hours. Laboratory confirmation is essential, obtained through detection of serum measles immunoglobulin M antibody or measles RNA on real-time polymerase chain reaction testing of a respiratory specimen. A serum sample and a throat or nasopharyngeal swab should be collected. Additionally, a urine sample may be collected because urine may contain the measles virus.1 Information on specimen collection is available on the CDC website at https://www.cdc.gov/measles/lab-tools/index.html.
The live attenuated measles vaccine became available in the United States in 1963, with an ineffective inactivated vaccine that was available until 1967. Vaccination that follows recommended guidelines is 97% effective in preventing viral transmission.1
MMR vaccination is recommended in children at 12 to 15 months and four to six years of age with at least 28 days between doses. Students at post–high school educational institutions, adults without evidence of immunity, and international travelers six months and older should also be immunized. Health care personnel should have documented evidence of immunity.1
Adults born before 1957 are considered immune from measles. Others are considered immune if they have laboratory evidence of immunity; confirmation of previous measles; or documentation of adequate (live) vaccination with at least one dose of a measles-containing vaccine administered on or after the first birthday for preschool-aged children and adults not at high risk or two doses for school-aged children and adults at higher risk, such as college students, health care workers, and international travelers.1 Those who received a dose of measles vaccine between 1963 and 1967 should be tested for immunity or revaccinated. Assessing immune status is especially important for children, adolescents, and adults born between 1957 and 1967.
An important misconception about the MMR vaccine is that it is linked to autism, which was falsely reported in a 1998 Lancet article. The article was retracted in 2010 after unethical conduct by the author and reports of fraudulent data were uncovered.6 The MMR vaccine is safe. Numerous large studies show no link between the MMR vaccine and autism.7–9 Additionally, a Cochrane review found no association between the MMR vaccine and autism, leukemia, asthma, hay fever, type 1 diabetes mellitus, gait disturbance, Crohn disease, demyelinating diseases, or bacterial or viral infections.10
All 50 states have legislation requiring specified vaccines for students, including the MMR vaccine, with some exemptions granted for medical reasons. Medical contraindications to the MMR vaccine include a history of severe allergic reaction to the vaccine or its components, known severe immunodeficiency (e.g., from hematologic and solid tumors, chemotherapy, congenital immunodeficiency, long-term immunosuppressive therapy, HIV infection), and family history of altered immunocompetence.11
Religious exemptions are granted by 45 states and the District of Columbia, and 18 states allow philosophical (personal) exemptions. New York and Maine enacted legislation in 2019, joining California, Mississippi, and West Virginia, in allowing only medical vaccine exemptions, a policy supported by the American Academy of Family Physicians.12,13 Unfortunately, many states are increasingly allowing non-medical exemptions.3,14
Family physicians should familiarize themselves with their states' pending legislation and consider getting involved in legislative advocacy, condone only medical vaccine exemptions, and give a strong vaccination recommendation to patients without immunity to help prevent the spread of measles.