
Am Fam Physician. 2019;100(9):530-531
Author disclosure: No relevant financial affiliations.
The allopurinol hypersensitivity assay, or HLA-B*58:01 test, is a blood test to detect the presence of a human leukocyte antigen B (HLA-B) genetic variant that increases the risk of life-threatening, severe cutaneous adverse reactions in patients taking allopurinol. Although the HLA-B*58:01 test has not been approved by the U.S. Food and Drug Administration, the American College of Rheumatology and the Clinical Pharmacogenetics Implementation Consortium recommend it for select populations before initiation of allopurinol therapy.1,2
Accuracy
A systematic review including 7,534 patients found that the HLA-B*58:01 test is 78% sensitive and 96% specific, with a negative predictive value of 99% for the development of severe cutaneous adverse reactions associated with allopurinol treatment. Most patients in the study were Asian. No studies from America, India, or Africa were identified.3 Depending on the technology used, it may take up to three weeks to receive test results.4 The absence of the HLA-B*58:01 allele does not eliminate the risk of severe cutaneous adverse reactions because additional factors such as renal impairment and concurrent thiazide use have also been shown to increase risk.1
Benefit
In patients with the HLA-B*58:01 allele, the odds ratio for the development of a severe cutaneous adverse reaction is 74 (95% CI, 27 to 204) in Asians and 101 (95% CI, 45 to 229) in non-Asians.5,6 These reactions occur at a baseline rate of 0.69 per 1,000 person-years among allopurinol users in the United States. Patients hospitalized with severe cutaneous adverse reactions in the United States have a mortality rate of 27%.5
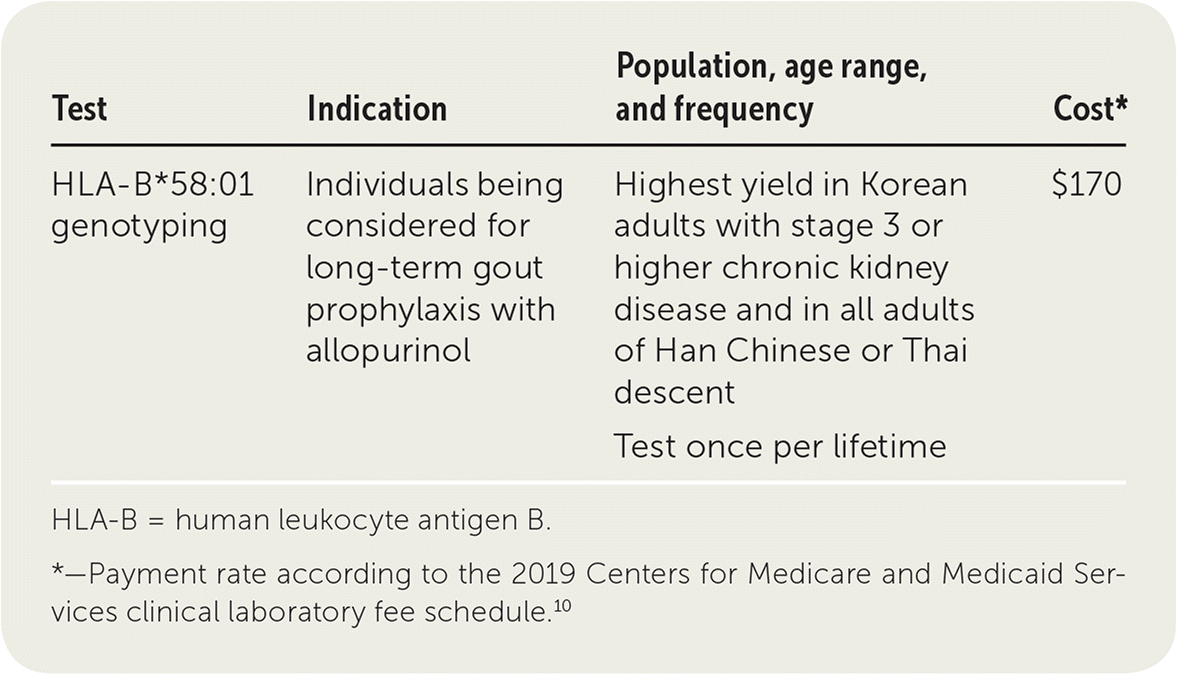
| Test | Indication | Population, age range, and frequency | Cost* |
|---|---|---|---|
| HLA-B*58:01 genotyping | Individuals being considered for long-term gout prophylaxis with allopurinol | Highest yield in Korean adults with stage 3 or higher chronic kidney disease and in all adults of Han Chinese or Thai descent | $170 |
| Test once per lifetime |
In a prospective study, 2,910 Taiwanese patients with recurrent gout underwent HLAB*58:01 testing before taking allopurinol. The 571 patients (19.6%) who tested positive for the allele were prescribed an alternative medication. The 2,339 patients (80.4%) who tested negative were treated with allopurinol. Of the patients treated with allopurinol, none experienced severe cutaneous adverse reactions, although seven reactions would have been expected based on historical incidence among patients treated with allopurinol.7
Results of HLA-B*58:01 testing are likely to change treatment options because an alternative to allopurinol, such as febuxostat (Uloric) or probenecid, is recommended in patients who are carriers of this allele. Populations with higher prevalence of the allele are more likely to benefit from pretreatment testing.3
Allopurinol-induced severe cutaneous adverse reactions are most likely to occur during initiation of treatment. Testing is not necessary in patients who have tolerated allopurinol for longer than three months.3
Harms
Because allopurinol is not prescribed to patients who test positive for the HLA-B*58:01 allele, about one in five patients tested would be exposed to the additional cost and harms of alternative medications, such as febuxostat, which increases all-cause and cardiovascular mortality (number needed to harm over 2.7 years = 90 and 71, respectively).8 Patients who test positive for the allele may also experience the effects of undertreatment, such as more frequent acute gout attacks, joint damage, or chronic synovitis, if alternatives to allopurinol are not affordable or available.
Cost
A cost-effectiveness analysis compared universal pretreatment screening in the United States with no testing, with positive individuals receiving febuxostat.9 Prevalence of the HLA-B*58:01 allele was 0.7% among whites and Hispanics, 3.8% among blacks, and 7.4% among Asians. The universal testing strategy was suggested to be cost-effective for blacks and Asians but not for whites or Hispanics based on a higher-than-conventional threshold of $109,000 per quality-adjusted life-year. This analysis used the 2016 Centers for Medicare and Medicaid Services clinical laboratory fee schedule of $129. The cost of the HLA-B*58:01 test in 2019 is about $170.10
Bottom Line
To prevent severe cutaneous adverse reactions, specialty organizations recommend that clinicians consider HLA-B*58:01 testing in Korean adults with stage 3 or higher chronic kidney disease and in all adults of Han Chinese or Thai descent before initiating allopurinol therapy.1,2 In other patients, this test is not likely to be cost-effective, and the net benefit is not known.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Air Force Medical Department or the U.S. Air Force at large.
