Am Fam Physician. 2020;101(1):online
Author disclosure: No relevant financial affiliations.

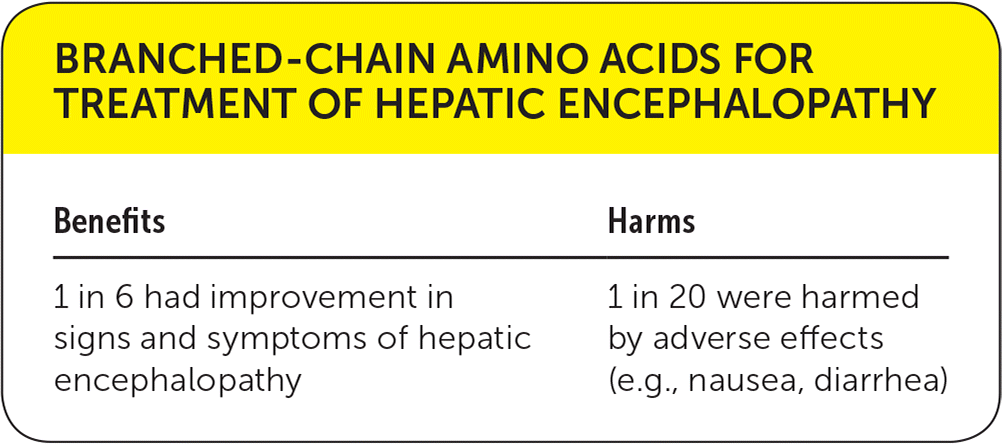
| Benefits | Harms |
|---|---|
| 1 in 6 had improvement in signs and symptoms of hepatic encephalopathy | 1 in 20 were harmed by adverse effects (e.g., nausea, diarrhea) |
Details for This Review
Study Population: Adults with hepatic encephalopathy and cirrhosis, mostly caused by alcoholic liver disease or viral hepatitis
Efficacy End Points: Mortality, hepatic encephalopathy (number with improvement)
Harm End Points: Gastrointestinal symptoms, including nausea and diarrhea; other reported adverse events
Narrative: Hepatic encephalopathy 1 is a brain dysfunction that can be mild with minimal confusion, or overt and severe with coma.1–3 Cirrhosis, where scar tissue replaces normal hepatic tissue, is the most common cause of hepatic encephalopathy. Branched-chain amino acids (BCAAs) may reduce hepatic encephalopathy by helping skeletal muscle detoxify blood4–6 and are, therefore, recommended by some as routine treatment.2,3
The systematic review summarized here identified 16 randomized trials of 827 patients with hepatic encephalopathy (97% with cirrhosis). Primary outcomes were mortality, hepatic encephalopathy (number with improvement), and harms. Patients were followed for varying time periods ranging from one to 104 weeks.1
BCAAs had benefits in improving signs and symptoms of hepatic encephalopathy assessed mainly by the West Haven Criteria (relative risk [RR] = 0.7; 95% CI, 0.6 to 0.9; 17% absolute risk difference [ARD]; number needed to treat = 6 [for every six patients treated, one measurably improved]). However, no effect was found on mortality (RR = 0.9; 95% CI, 0.7 to 1.1). BCAAs also increased nausea and diarrhea (RR = 3.4; 95% CI, 0.7 to 16.5; 5% ARD; number needed to harm = 20), but no serious adverse events were reported.1
The West Haven Criteria (https://www.mdcalc.com/hepatic-encephalopathy-grades-stages) classifies the degree of mental status disturbance in encephalopathy using a 4-point scoring system ranging from reversal of sleep patterns and mild alteration in cognition to deep coma.2 The portal-systemic encephalopathy (PSE) index may also objectively describe the overall clinical severity of hepatic encephalopathy.7 It is calculated following the assessment of five elements: mental status (evaluated using the West Haven Criteria), presence and intensity of asterixis, time taken to complete psychometric tests of intellectual function (such as a number connection test), venous ammonia level, and abnormalities on electroencephalography.7
In this meta-analysis, six studies (Cerra 1985, Hwang 1988, Muto 2005, Strauss 1986, Vilstrup 1990, and Rossi-Fanelli 1986) assessed improvement of hepatic encephalopathy strictly by West Haven Criteria scoring only.1 The other 10 studies (Fiaccadori 1984, Horst 1984, Michel 1985, Egberts 1985, Calvey 1985, Marchesini 1990, Hayashi 1991, Plauth 1993, Les 2011, and Marchesini 2003) included the West Haven Criteria to evaluate the degree of encephalopathy, and some or all components of the PSE index.1
Caveats: The authors of the Cochrane review graded the evidence as high quality for hepatic encephalopathy. However, there was moderate heterogeneity of their results, which raises concerns about validity and applicability. In addition, most trials were nonblinded, small, and judged to be at high risk of bias. The ‘high-quality’ grade seems debatable; and a large, well-done clinical trial may upend these findings.
The review also pooled trials using different forms of BCAAs.1 Nine trials assessed oral administration and seven assessed intravenous administration, with only oral administration showing a statistical benefit. In addition, assessing hepatic encephalopathy is fraught with subjectivity and disagreement. For instance, many studies in this review used the PSE index, which the U.S. Food and Drug Administration has rejected as inadequate, mainly because of the inclusion of blood ammonia levels and severity of asterixis.8 The utility of ammonia level is controversial given that ammonia concentration is not useful for screening for hepatic encephalopathy because levels vary if they are arterial or venous.9,10 These levels may be significantly affected by collection techniques and may be falsely elevated if the sample was collected after fist clenching or using a tourniquet, or if the sample was not placed on ice.10 Also, asterixis is not specific to hepatic encephalopathy because it can also be observed in patients with other forms of metabolic encephalopathies such as uremia and respiratory failure.10
Without blinded assessors, a method not used in most of these trials, and a validated scale, it is difficult to have confidence in these findings. This is particularly true when the only objective outcome (mortality) showed no difference between groups. The recommendation of yellow (unclear benefits) is based on inconclusive data supporting the benefits of BCAAs for hepatic encephalopathy.