Am Fam Physician. 2020;101(1):44-46
Author disclosure: No relevant financial affiliations.
The PLAC test is a blood test used to measure serum activity of lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme that breaks down oxidized low-density lipoprotein in the vascular wall. Higher levels of Lp-PLA2 activity are thought to promote atherosclerotic plaque formation.1 The PLAC test was approved by the U.S. Food and Drug Administration for this indication in 2014.2
Accuracy
In a prospective multiethnic cohort of 5,456 asymptomatic patients followed over a median of 10.2 years, Lp-PLA2 activity was associated with an increased risk of incident cardiovascular disease. Patients included persons with baseline subclinical vascular disease (n = 3,228 [450 events]; hazard ratio [HR] = 1.17 [95% CI, 1.06 to 1.30]) and without subclinical disease (n = 2,228 [66 events]; HR = 1.30 [95% CI, 1.01 to 1.68]), as determined by coronary artery calcium score and carotid intima-medial thickness. These associations persisted after adjustment for age, sex, race, and ethnicity. However, when further adjusted for body mass index, diabetes mellitus, smoking status, education, systolic blood pressure, use of antihypertensive medications, total and high-density lipoprotein cholesterol, use of lipid-lowering medications, and C-reaction protein, the associations were no longer significant.3 The analysis suggested that Lp-PLA2 activity does not add significant information to the standard cardiovascular risk assessment.
Contrast this with a 2010 collaborative analysis of 18 prospective studies of Lp-PLA2 activity. A subanalysis (12 studies), representing a heterogeneous population of 41,121 patients with and without a history of vascular disease and a total of 3,963 fatal and nonfatal cardiovascular events, showed an increased risk of coronary heart disease (CHD) per one standard deviation higher Lp-PLA2 activity (risk ratio [RR] = 1.10 [95% CI, 1.05 to 1.16]). This association persisted after adjusting for standard risk factors. Other subanalyses showed a similar risk-adjusted association between Lp-PLA2 activity and vascular death (n = 38,105 [nine studies; 2,689 events]; RR = 1.16 [95% CI, 1.09 to 1.24]) and nonvascular death (n = 37,084 [eight studies; 2,795 events]; RR = 1.10 [95% CI, 1.04 to 1.17]).4
There is less evidence to support risk assessment using the manufacturer’s suggested binary cut-off of 225 nmol per minute per mL or higher to define high Lp-PLA2 activity.5,6 The validation study referenced on the test website and package insert is a published abstract containing only a summary of data from a subanalysis case-cohort study (n = 5,025; including 771 participants with incident CHD and 4,254 without CHD) sampled from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) trial.7,8 The abstract reports HRs for CHD using sex-specific cut-offs for elevated Lp-PLA2 activity (defined as greater than 80th percentile): 250 nmol per minute per mL for men and 200 nmol per minute per mL for women. Given that statistically significant differences in Lp-PLA2 activity between men and women have been demonstrated elsewhere, it is unclear why the PLAC test does not also have sex-specific cut-offs.9,10
A subanalysis of the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial suggested that the PLAC test binary cut-off is neither sensitive nor specific as a marker of CHD. Baseline Lp-PLA2 activity levels were measured in 10,439 patients randomized to placebo or rosuvastatin (Crestor) for primary prevention. Lp-PLA2 activity levels were categorized in quartiles, with the 4th quartile representing Lp-PLA2 activity levels greater than 222 nmol per minute per mL, similar to the manufacturer’s cut-off of 225 nmol per minute per mL. Among the patients randomized to placebo (n = 5,446; 149 primary events at one year of follow-up), the sensitivity and specificity of a baseline Lp-PLA2 activity in the 4th (highest) quartile were 37% and 75%, respectively. This translates to a positive likelihood ratio (LR+) of 1.48 and a negative likelihood ratio (LR–) of 0.8. Among the patients randomized to rosuvastatin (n = 5,496; 80 events), the sensitivity and specificity were similar, at 31% and 75%; LR+ = 1.24; LR– = 0.92.11
Benefit
There is no clear benefit to using the PLAC test as a binary indicator of high risk or low risk (less than 225 nmol per minute per mL) in addition to other standard cardiovascular risk markers. No studies have demonstrated improved outcomes from using the PLAC test to reclassify a patient from a lower to higher cardiovascular risk level.
Although there is some short-term (one year of follow-up) evidence that pravastatin (Pravachol)-mediated reduction of Lp-PLA2 activity may have predictive value for future CHD events, the JUPITER trial did not show any predictive benefit of measuring Lp-PLA2 activity after statin treatment.11,12 Trials of the direct Lp-PLA2 inhibitor (darapladib) have not shown improvements in patient-oriented cardiovascular outcomes.13
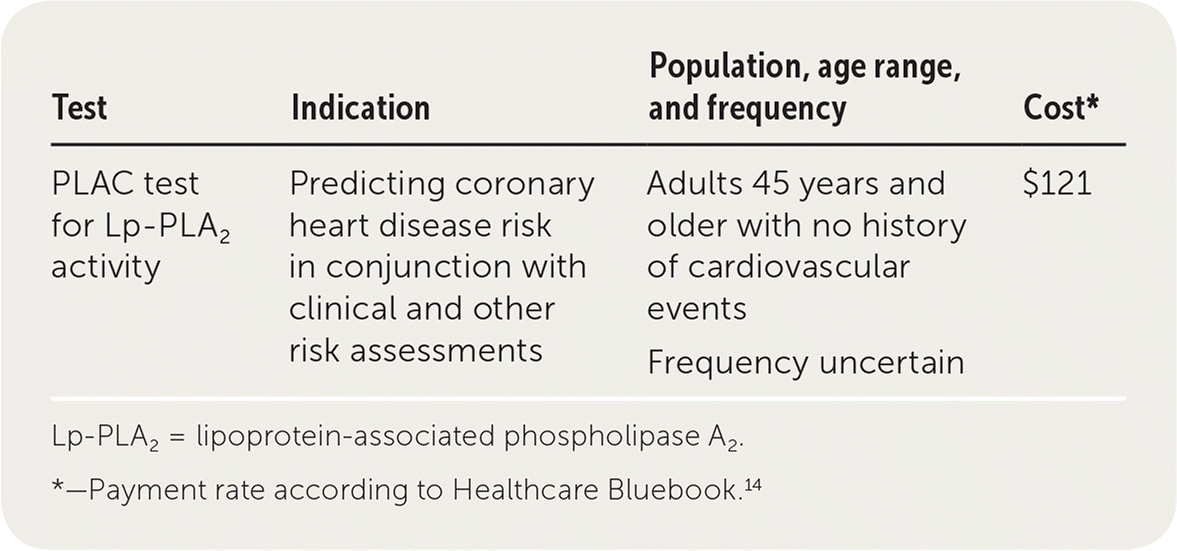
| Test | Indication | Population, age range, and frequency | Cost* |
|---|---|---|---|
| PLAC test for Lp-PLA2 activity | Predicting coronary heart disease risk in conjunction with clinical and other risk assessments | Adults 45 years and older with no history of cardiovascular events Frequency uncertain | $121 |
Harms
Given the lack of consistent patient-oriented outcomes from use of PLAC in risk assessment, as well as the sex-specific differences in Lp-PLA2 activity that are not accounted for in the test cut-off, there is potential for harm in using it to reclassify a patient’s cardiovascular risk. A high PLAC score might lead to the unnecessary and potentially harmful cardiac testing for further risk stratification, or to unnecessary use of statin medication. Conversely, a low PLAC score might falsely reassure a patient and clinician, thus resulting in underutilization of statins and other measures for primary prevention of cardiovascular events.
Cost
Bottom Line
The PLAC test for Lp-PLA2 activity provides little additional value beyond standard cardiovascular risk assessment, and any potential benefits are likely outweighed by harms. Current guidelines do not include the PLAC test nor other tests for Lp-PLA2 activity in risk assessment.16,17 Other tests with greater utility that are supported by the American College of Cardiology/American Heart Association guidelines for primary prevention include high-sensitivity C-reaction protein, lipoprotein(a), apolipoprotein B, ankle-brachial index, and the coronary artery calcium score.16