Am Fam Physician. 2020;101(4):230-232
Author disclosure: No relevant financial affiliations.
Clinical Question
Can signs, symptoms, and simple tests be used to determine the likelihood of heart failure with reduced ejection fraction?
Evidence Summary
Considerations for the selection and implementation of any clinical prediction rule include whether it has been validated in a new population, whether it has sufficient accuracy to be trustworthy, whether the resulting risk categories are clinically useful (i.e., the low-risk group is low enough to comfortably rule out the condition), whether it is practical to implement in your setting, and whether the spectrum of illness is similar to that in your population. For the diagnosis of heart failure with reduced ejection fraction, the spectrum of disease and its presentation may be different in the primary care setting compared with the emergency department, as is the availability of blood tests such as brain natriuretic peptide (BNP).
A systematic review recently identified nine previously published clinical prediction rules1; one additional clinical prediction rule was identified in an abbreviated search by the author.2 Clinical prediction rules were excluded if they had not been prospectively and externally validated in a new population or if they were conducted in a limited population, such as only patients with diabetes mellitus or chronic lung disease, leaving four studies for consideration.2–5 Of these, three were developed in the primary care setting3,4 or studied patients identified in the primary care setting,5 and one was developed in the emergency department setting.2
The rule by Fahey and colleagues recruited primary care patients with clinically suspected heart failure and used five variables (male sex, orthopnea, history of myocardial infarction, presence of jugular venous distension, and abnormal findings on electrocardiography [ECG]). The resulting risk score had a broad range of risk categories from 0% (none of the factors present) to 97% (all five present).3 However, in a prospective validation, it significantly underestimated the risk of heart failure with reduced ejection fraction, partly because of a much higher likelihood of heart failure in the validation population.
Kelder and colleagues developed a 10-item clinical prediction rule in patients with clinically suspected heart failure with reduced ejection fraction (Table 1).4 The rule identified four risk groups from 4.7% to 87.5%.4 It was prospectively validated in a second study of patients 80 years and older and using a cutoff of more than 54 points for referral. This cutoff was 81% sensitive and 57% specific, which is similar to the cutoff in the original study.6 However, this rule requires use of N-terminal prohormone BNP testing, which is not widely available in the primary care setting. The Brest score was developed and validated in patients with new-onset dyspnea presenting to the emergency department and has 11 variables, including two ECG findings (Table 2).2
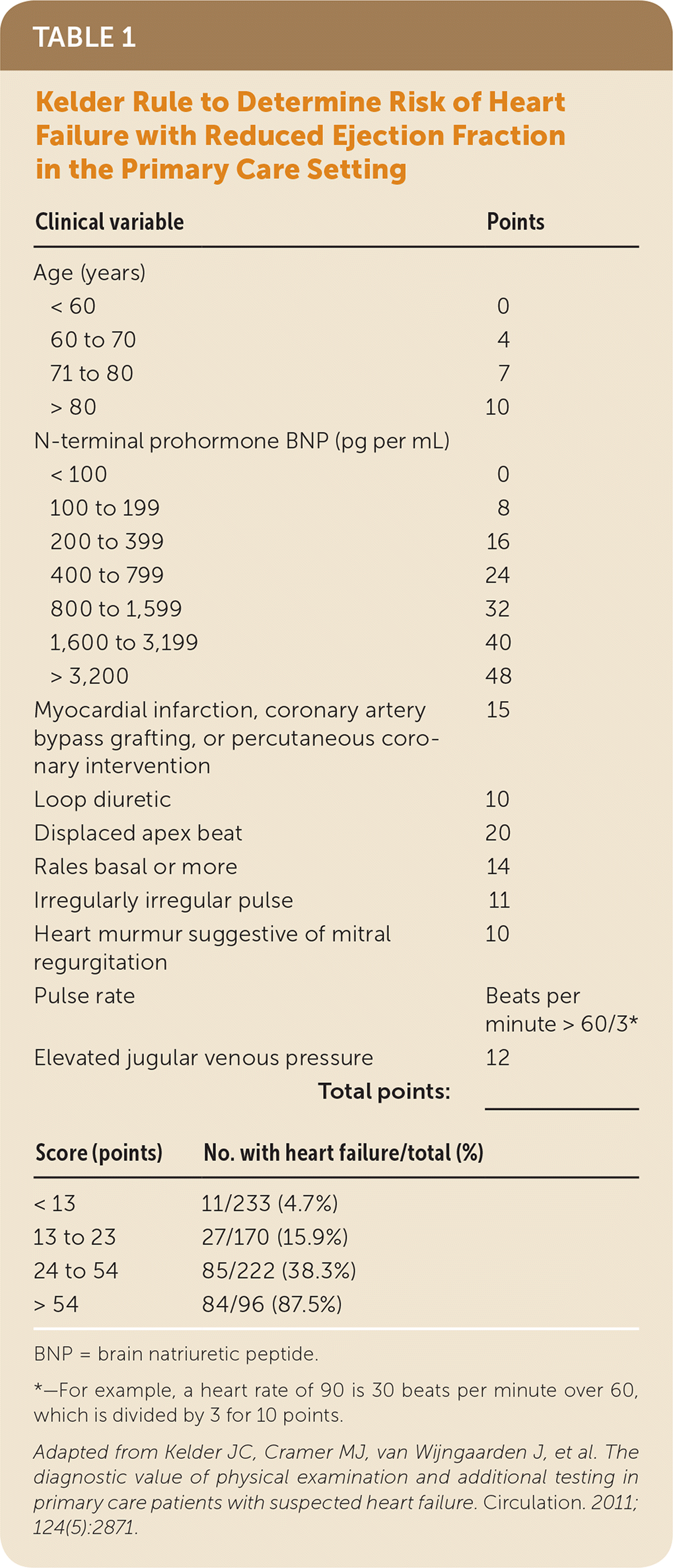
| Clinical variable | Points | |
|---|---|---|
| Age (years) | ||
| < 60 | 0 | |
| 60 to 70 | 4 | |
| 71 to 80 | 7 | |
| > 80 | 10 | |
| N-terminal prohormone BNP (pg per mL) | ||
| < 100 | 0 | |
| 100 to 199 | 8 | |
| 200 to 399 | 16 | |
| 400 to 799 | 24 | |
| 800 to 1,599 | 32 | |
| 1,600 to 3,199 | 40 | |
| > 3,200 | 48 | |
| Myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention | 15 | |
| Loop diuretic | 10 | |
| Displaced apex beat | 20 | |
| Rales basal or more | 14 | |
| Irregularly irregular pulse | 11 | |
| Heart murmur suggestive of mitral regurgitation | 10 | |
| Pulse rate | Beats per minute > 60/3* | |
| Elevated jugular venous pressure | 12 | |
| Total points: | _______ | |
| Score (points) | No. with heart failure/total (%) | |
| < 13 | 11/233 (4.7%) | |
| 13 to 23 | 27/170 (15.9%) | |
| 24 to 54 | 85/222 (38.3%) | |
| > 54 | 84/96 (87.5%) | |
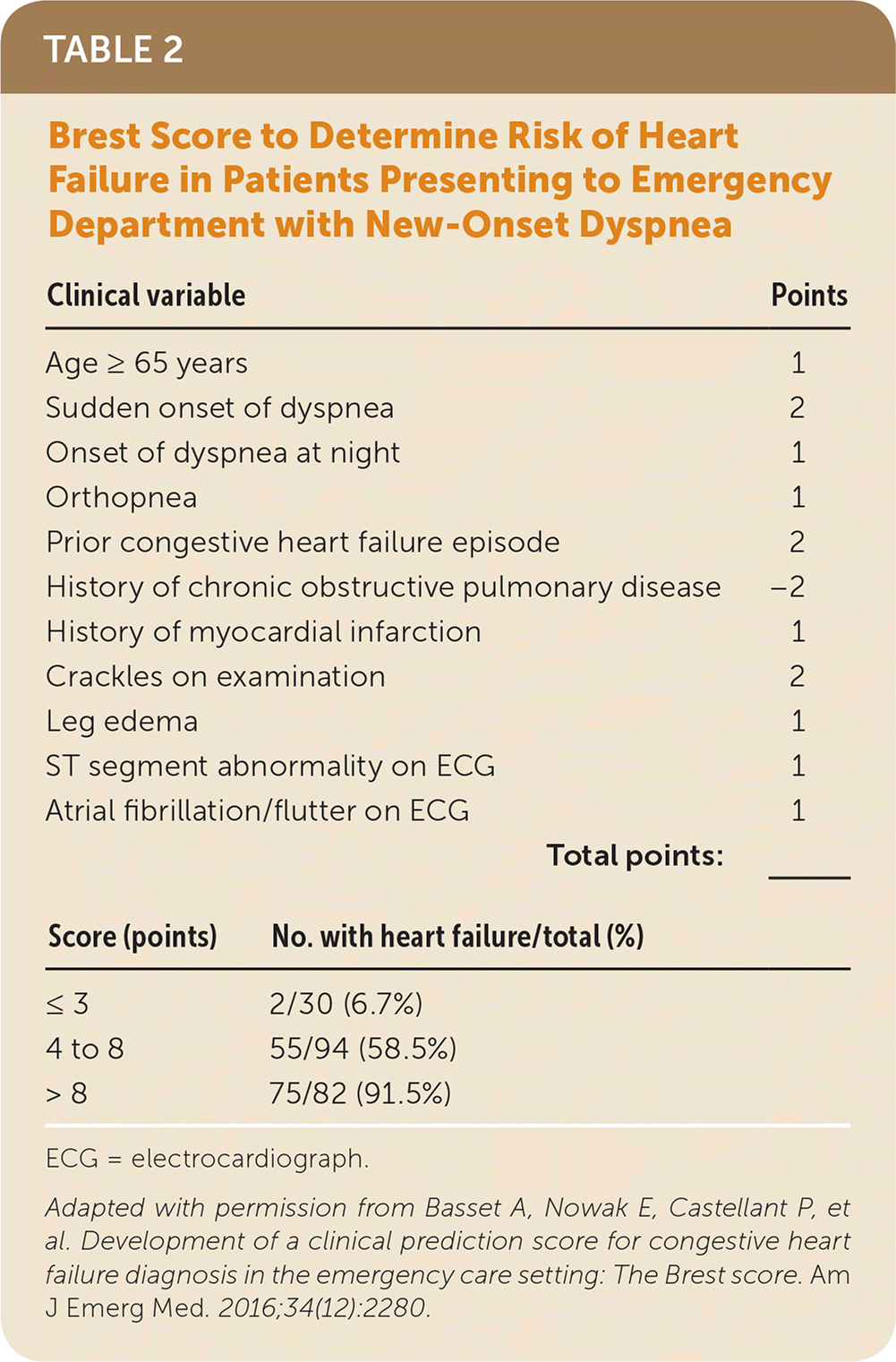
| Clinical variable | Points | |
|---|---|---|
| Age ≥ 65 years | 1 | |
| Sudden onset of dyspnea | 2 | |
| Onset of dyspnea at night | 1 | |
| Orthopnea | 1 | |
| Prior congestive heart failure episode | 2 | |
| History of chronic obstructive pulmonary disease | −2 | |
| History of myocardial infarction | 1 | |
| Crackles on examination | 2 | |
| Leg edema | 1 | |
| ST segment abnormality on ECG | 1 | |
| Atrial fibrillation/flutter on ECG | 1 | |
| Total points: | _______ | |
| Score (points) | No. with heart failure/total (%) | |
| ≤ 3 | 2/30 (6.7%) | |
| 4 to 8 | 55/94 (58.5%) | |
| > 8 | 75/82 (91.5%) | |
Finally, Roalfe and colleagues obtained individual patient-level data from five primary care studies of patients evaluated for heart failure with reduced ejection fraction, and the data were used to develop a parsimonious model with three clinical variables plus BNP.5 Using this MICE (male, infarction, crepitations, edema) score (Table 35), echocardiography should be ordered for patients with acute dyspnea if there is a history of myocardial infarction or basal crepitations or the patient is a male with ankle edema. Otherwise, the physician should order a BNP test and then echocardiography if findings are abnormal. The MICE score had a sensitivity of 81% to 89% and specificity of 57% to 74% in prospective validation populations. This evaluation does not preclude the need to assess the patient for other causes of dyspnea, such as chest radiography to assess for pneumonia or pneumothorax.
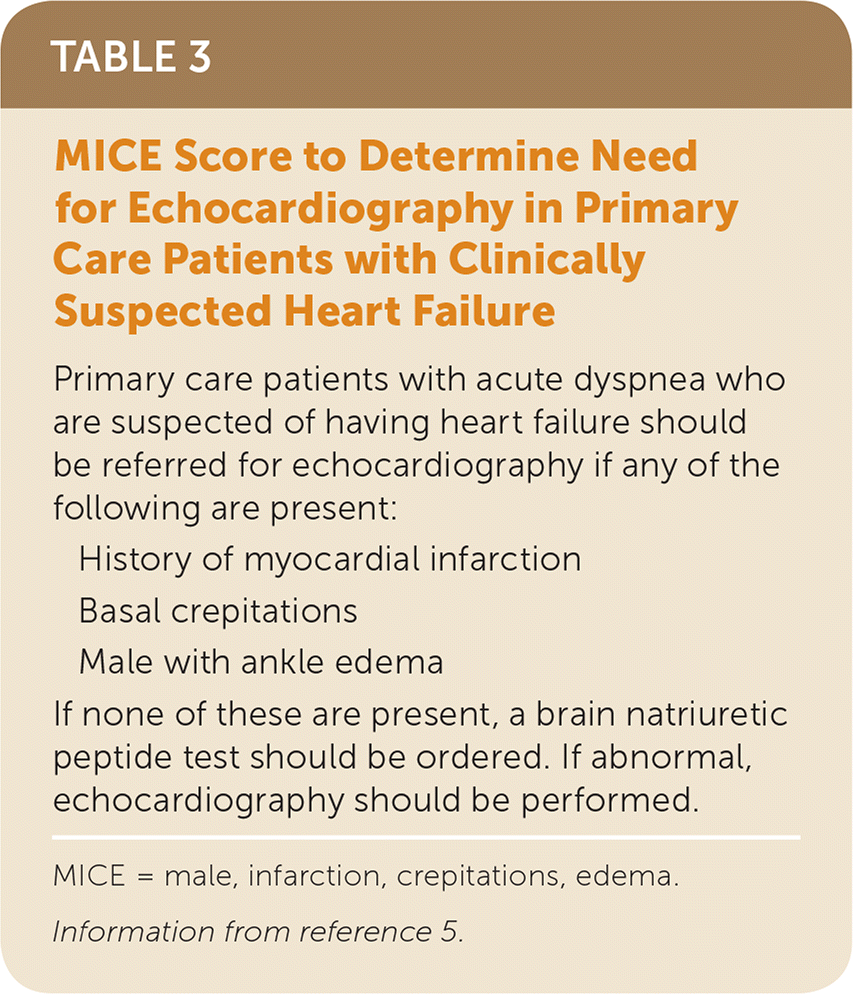
| Primary care patients with acute dyspnea who are suspected of having heart failure should be referred for echocardiography if any of the following are present: |
| History of myocardial infarction |
| Basal crepitations |
| Male with ankle edema |
| If none of these are present, a brain natriuretic peptide test should be ordered. If abnormal, echocardiography should be performed. |
Ultimately, the selection of the best clinical prediction rule to use depends on the setting and the availability of diagnostic tests. The MICE and Brest scores are best suited for the primary care setting because they do not require blood tests for the initial evaluation, with the caveat that the Brest score has been validated only in the emergency department. The Kelder rule is accurate and well validated but is more relevant in the emergency department because it requires the N-terminal prohormone BNP test.
Applying the Evidence
A 67-year-old woman presents with dyspnea with a gradual onset over the past two months and has basilar crackles and leg edema on examination. She has a history of myocardial infarction that occurred three years ago but has had no symptoms since that episode. The ECG findings are normal. Her Brest score is 5 (1 point for age, 1 for history of myocardial infarction, 2 for crackles, and 1 for leg edema), which is consistent with a 58.5% likelihood of heart failure. The MICE score confirms that you should refer her for urgent echocardiography because she has two of the three initial risk factors (history of myocardial infarction and basal crepitations). The echocardiogram confirms heart failure with an ejection fraction of 35%.