
Am Fam Physician. 2020;101(5):307-308
Author disclosure: No relevant financial affiliations.
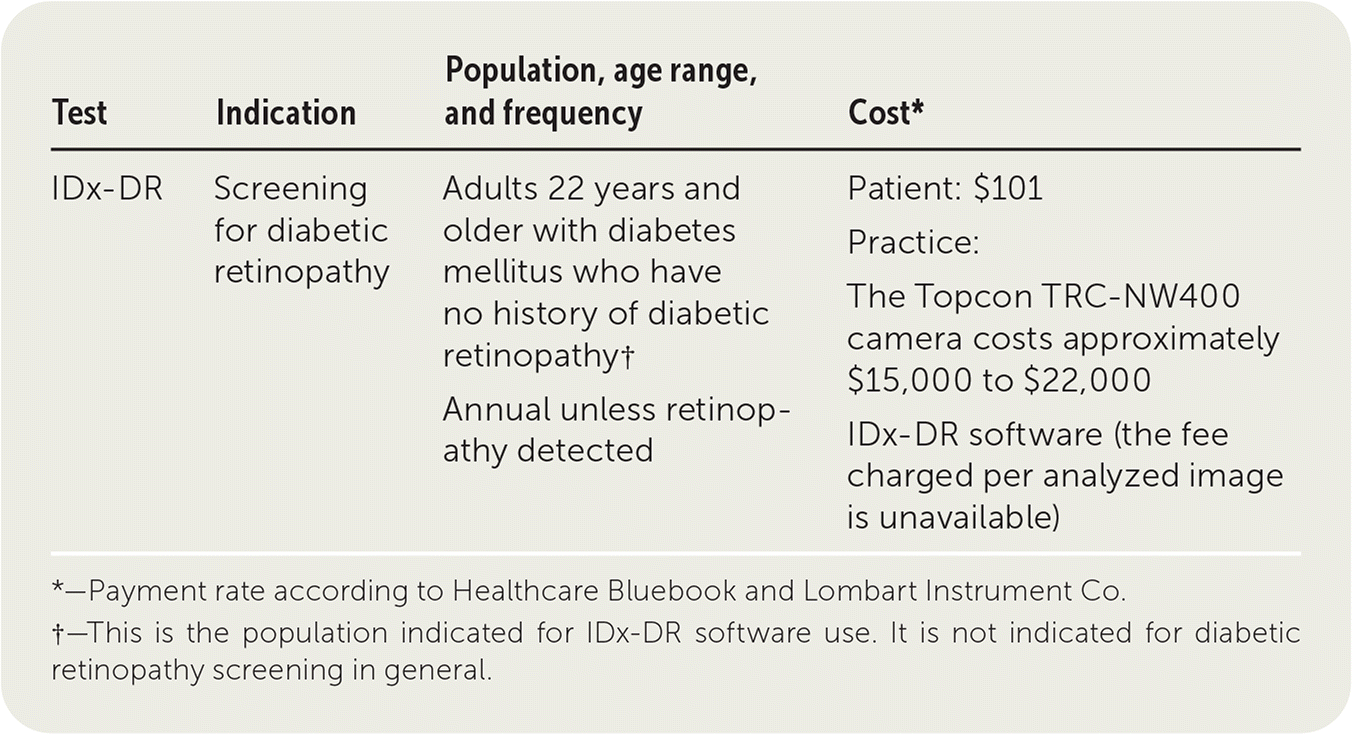
IDx-DR is a software program that uses artificial intelligence (AI) to analyze retinal images taken with the Topcon TRC-NW400, a fully automated nonmydriatic retinal camera designed to obtain high-resolution color images of the retina and the anterior segment of the eye. It is approved by the U.S. Food and Drug Administration for diabetic retinopathy screening in adults 22 years and older with diabetes mellitus.
| Test | Indication | Population, age range, and frequency | Cost* |
|---|---|---|---|
| IDx-DR | Screening for diabetic retinopathy | Adults 22 years and older with diabetes mellitus who have no history of diabetic retinopathy† Annual unless retinopathy detected | Patient: $101 Practice: The Topcon TRC-NW400 camera costs approximately $15,000 to $22,000 IDx-DR software (the fee charged per analyzed image is unavailable) |
Accuracy
Primary care practices can provide diabetic retinopathy screening recommended by the American Diabetes Association using automated digital fundal cameras to obtain images that are transmitted to an ophthalmologist for review.1 The IDx-DR system has been studied previously in international primary care clinics and research trials.2–4 The system used by the University of Wisconsin–Madison Fundus Photograph Reading Center was the reference standard for grading severity of diabetic retinopathy, using a standard protocol from the Early Treatment Diabetic Retinopathy Study.5
The IDx-DR system was studied in 819 adults with diabetes and no previous diagnosis of diabetic retinopathy from 10 primary care practices.6 IDx-DR correctly identified 173 of the 198 patients with more than minimal diabetic retinopathy, with a sensitivity of 87% (95% CI, 82% to 92%) and specificity of 90% (95% CI, 87% to 92%). At a 24% prevalence of minimal diabetic retinopathy, the positive predictive value is 73% (95% CI, 68% to 77%) and the negative predictive value is 96% (95% CI, 94% to 97%).
Benefit
The camera used in the IDx-DR system is easy to learn, and staff members can be taught to reliably obtain images without direct physician supervision. The images are transferred to the AI program electronically, and a result is produced within minutes, allowing identification of patients who require referral to an eye care professional at the time of the visit. It is an appealing option for practices that struggle to reach the recommended retinopathy screening quality measure (Centers for Medicare and Medicaid Services measure #117; target = 100%). Because the IDx-DR system does not initially rely on specialist intervention, it may reduce referral times in communities where the major barrier to care is limited access to specialist appointments. Patients with a negative result are advised to repeat the imaging and analysis in 12 months. If more than mild diabetic retinopathy is identified, the patient should be referred to an ophthalmologist for additional diagnostic evaluation. It is not known if the improved detection of diabetic retinopathy leads to earlier diagnosis and treatment or a reduction in vision complications from diabetes.
Harms
Examination with the IDx-DR system is not invasive and does not require dilation of the eye. The major potential harm is the risk of missing significant retinopathy based on a failure of the AI detection system. It is also possible for the system to generate unnecessary referrals because of false-positive results. The American Diabetes Association recommends a comprehensive eye examination, including retinal screening for patients with type 2 diabetes at diagnosis, and after five years for patients diagnosed with type 1 diabetes. Other serious eye conditions may be missed if patients or clinicians delay or defer the recommended comprehensive examination.
Cost
A fee is charged for each successfully analyzed image using the IDx-DR system, but a price is not currently available. The TRC-NW400 camera costs approximately $15,000 to $22,000.7
Use of the Current Procedural Terminology (CPT) code 92250 (bilateral fundus photography with interpretation and report) is recommended when patients are screened with the IDx-DR system. The 2019 Medicare Physician Fee Schedule allowable charge is $64.03. Of this amount, $38.35 is assigned to the technical component, and $25.68 is the value of the professional component (i.e., interpretation).8 These prices may vary based on local wage indices and Medicare's multiple procedure payment reduction. Reimbursement rates have decreased over the past few years. The reported fair price according to Healthcare Bluebook is $101.9
Bottom Line
IDx-DR is a reliable screening tool used to diagnose diabetic retinopathy in the primary care office and may reduce barriers to screening and improve gaps in eye care for those with diabetes. However, the up-front costs with declining reimbursements for office-based retinopathy screening may make implementing the system cost prohibitive for many primary care practices. It is not known whether improved detection of diabetic retinopathy will lead to a reduction in diabetes-related vision complications.
