
Am Fam Physician. 2020;101(12):721-729
Author disclosure: No relevant financial affiliations.
Despite dramatic reductions in the rates of bacteremia and meningitis since the 1980s, febrile illness in children younger than 36 months continues to be a concern with potentially serious consequences. Factors that suggest serious infection include age younger than one month, poor arousability, petechial rash, delayed capillary refill, increased respiratory effort, and overall physician assessment. Urinary tract infections are the most common serious bacterial infection in children younger than three years, so evaluation for such infections should be performed in those with unexplained fever. Abnormal white blood cell counts have poor sensitivity for invasive bacterial infections; procalcitonin and C-reactive protein levels, when available, are more informative. Chest radiography is rarely recommended for children older than 28 days in the absence of localizing signs. Lumbar puncture is not recommended for children older than three months without localizing signs; it may also be considered for those from one to three months of age with abnormal laboratory test results. Protocols such as Step-by-Step, Laboratory Score, or the Rochester algorithms may be helpful in identifying low-risk patients. Rapid influenza testing and tests for coronavirus disease 2019 (COVID-19) may be of value when those diseases are circulating. When empiric treatment is appropriate, suggested antibiotics include ceftriaxone or cefotaxime for infants one to three months of age and ampicillin with gentamicin or with cefotaxime for neonates. For children three months to three years of age, azithromycin or amoxicillin is recommended if pneumonia is suspected; for urinary infections, suggested antibiotics are cefixime, amoxicillin/clavulanate, or trimethoprim/sulfamethoxazole. Choice of antibiotics should reflect local patterns of microbial resistance.
The evaluation of children younger than 36 months presenting with fever has long been challenging for physicians. Beginning in the 1980s, guidelines were developed to identify children who can be safely followed at home, but no plan has proved entirely satisfactory. Since publication of the previous article on this topic in American Family Physician,1 changes in epidemiology, microbial resistance, and available diagnostic tests have led to changes in recommendations. This review addresses the evaluation and care of previously healthy febrile children; children who are immunocompromised, were born prematurely, or who have other preexisting illness should be evaluated on a case-by-case basis.
| Recommendation | Sponsoring organization |
|---|---|
| Avoid routine continuation of antibiotic therapy beyond 48 hours for initially asymptomatic infants without evidence of bacterial infection. | American Academy of Pediatrics |
Epidemiology
The incidence of bacteremia and meningitis has decreased markedly since vaccines for Streptococcus pneumoniae and Haemophilus influenzae type B (Hib) infections were introduced. The incidence of invasive pneumococcal disease in children younger than five years dropped by more than 90% after the initiation of pneumococcal conjugate vaccines.2 Incidence of meningitis caused by Hib infections in children younger than five years fell by more than 99% in the first eight years following the introduction of the vaccine in the United States.3
The use of intrapartum prophylaxis against group B Streptococcus has increased, and rates of invasive group B streptococcal infection within the first week of life have dropped from 0.7 per 1,000 births in 19974 to 0.24 per 1,000 births in 2016.5 Illnesses caused by Listeria are also declining, coincident with widespread group B Streptococcus prophylaxis.6 An estimate of the U.S. rate of listeriosis suggests fewer than three confirmed diagnoses per year on average nationwide for neonates from days 7 through 28 of life and fewer than two confirmed diagnoses per year from days 29 through 364 of life.7 Coronavirus disease 2019 (COVID-19) should also be considered in the differential diagnosis of a child with fever, although most children will have relatively mild symptoms. The incidence of urinary tract infections (UTIs) is increasing, as is resistance of the causative organisms to ampicillin.8–11 UTIs are currently the most common serious bacterial infection among children 36 months and younger.11 Overall, rates of occult bacteremia with any organism, formerly 3% to 12%,12,13 are now less than 2%.8,14,15
Definition of Age Groups and Fever
Recommendations for the evaluation and treatment of fever in children generally use three different age groups: neonates from birth to 28 or 30 days of age,16,17 young infants one to three months of age,8,11,15,18–26 and older infants and young children three to 36 months of age.11 For the purpose of this article, we use younger than one month, one to three months, and three to 36 months.
Although some have used other thresholds to define a clinically significant fever,27 it is generally considered to be 38°C (100.4°F) or higher. In recent years, peripheral thermometers such as tympanic membrane and temporal artery devices have become more common. However, these methods are less accurate, and central thermometers such as the rectal thermometer are still preferred for assessing fever.28–30
History and Physical Examination
The history and physical examination of children with fever are used to recognize serious illness. Children with known immunocompromise, prematurity, or other significant medical history will need more extensive evaluation. If no localizing signs (e.g., difficulty breathing, diarrhea, foul-smelling urine) are present, diagnostic efforts must be directed toward determining an occult cause. Occult bacteremia and meningitis are more likely in younger patients who have not yet developed localizing responses.
Studies show that physicians’ global assessment that a child is ill-appearing is a valuable clinical predictor of serious bacterial infection,31,32 with a positive likelihood ratio greater than 5.0.31 Other predictors of serious bacterial infection include capillary refill of more than three seconds,31,33,34 poor arousability, petechial rash,32 and increased respiratory effort.31 No single clinical sign has a negative likelihood ratio less than 0.2 for excluding serious bacterial infection.31,32 Normal blood pressure readings are not necessarily reassuring because children often maintain normal blood pressure even in late stages of shock.35
Laboratory Testing and Imaging
One of the key challenges in the management of febrile children is the appropriate selection of imaging and laboratory tests. Inappropriate diagnostics may be misleading, painful, and expensive.
URINALYSIS AND URINE CULTURE
UTI is diagnosed by the presence of both pyuria and bacteriuria.19 Pyuria is defined by at least five white blood cells per high-power field on a centrifuged specimen or 10 white blood cells per mm3 on enhanced urinalysis.19 Urine culture is considered positive when growth is greater than 50,000 colony-forming units per mL for samples obtained by catheterization or suprapubic aspiration.19 The reference standard diagnostic test, suprapubic aspiration, can be difficult to perform, with procedural success rates between 23% and 90%.36 Samples obtained by bladder catheterization are 95% sensitive and 99% specific for diagnosing UTI, and the procedure has a higher success rate than suprapubic aspiration.36
Guidelines from the American Academy of Pediatrics recommend obtaining urine samples with suprapubic aspiration or bladder catheterization when the risk of UTI is high or if immediate antimicrobial therapy is being considered. The samples should be sent for urinalysis and culture.19 Urine obtained by bag or clean catch that tests positive for leukocyte esterase or nitrites may be enough to make the preliminary diagnosis of UTI,19 but a sample for urinalysis and urine culture should be obtained before starting antibiotics.8,19 In patients with a low risk of UTI, urine can be obtained by bag or clean catch and sent for dipstick testing and urinalysis.19 If these samples are consistent with infection, then sampling with aspiration or catheterization should be performed, and the sample should be sent for urinalysis and culture. When the risk of UTI is extremely low and immediate antimicrobial therapy is not needed, the patient can be observed without collecting a urine specimen.19 If UTI with fever is demonstrated in children younger than two years, follow-up should include renal and bladder ultrasonography to assess for anatomic abnormalities.37 Voiding cystourethrography should be performed for a patient’s first febrile UTI if renal and bladder ultrasonography findings suggest high-grade reflux or obstructive uropathy.19
Although UTIs are the most common serious bacterial infection in children presenting with fever, not all children with UTI develop fever. The Diagnosis of Urinary Tract Infection in Young Children study suggests that a urine sample should be obtained in any child younger than five years with three of the following features: pain or crying with urination, foul-smelling urine, previous UTI, signs of severe illness, and absence of severe cough.38,39
BLOOD CELL COUNTS
White blood cell counts and neutrophil counts have been included in algorithms for distinguishing low-risk patients.40,41 More recent studies have found that cell counts have poor sensitivity for detecting invasive bacterial infections (bacteremia or meningitis) in patients younger than 60 days.18,42 Leukocytosis is common with invasive Hib and with pneumococcal bacteremia, but these infections are now less common. Newer algorithms de-emphasize the complete blood count, using only the absolute neutrophil count43 or omitting cell counts entirely.26
BLOOD CULTURES
Blood cultures are recommended for evaluation of febrile neonates younger than one month because occult bacteremia is more common in this age group.11,14,22 For non–ill-appearing infants one to three months of age, there is no consensus as to whether blood cultures should be obtained. In children three months and older without localizing signs or a toxic appearance, blood cultures are 100 times more likely to grow contaminant species than pathogens.11,44 Therefore, the use of blood cultures in children at least three months of age is discouraged unless such indications are present.11,44
INFLAMMATORY MARKERS
Inflammatory biomarkers procalcitonin (PCT) and C-reactive protein (CRP) are associated with serious illness in young children. PCT measurements have several desirable features: They are not affected by administration of nonsteroidal anti-inflammatory drugs as CRP is,45 and PCT values rapidly increase beyond the normal range after the development of fever in patients with serious bacterial infection.17 When these two biomarkers are used together, CRP may identify some ill children in whom PCT levels are normal43 (Table 142). The high sensitivity of these tests and resulting low negative likelihood ratios indicate that a patient who has reassuring PCT and CRP results has a very low risk of UTI or other serious bacterial infection.
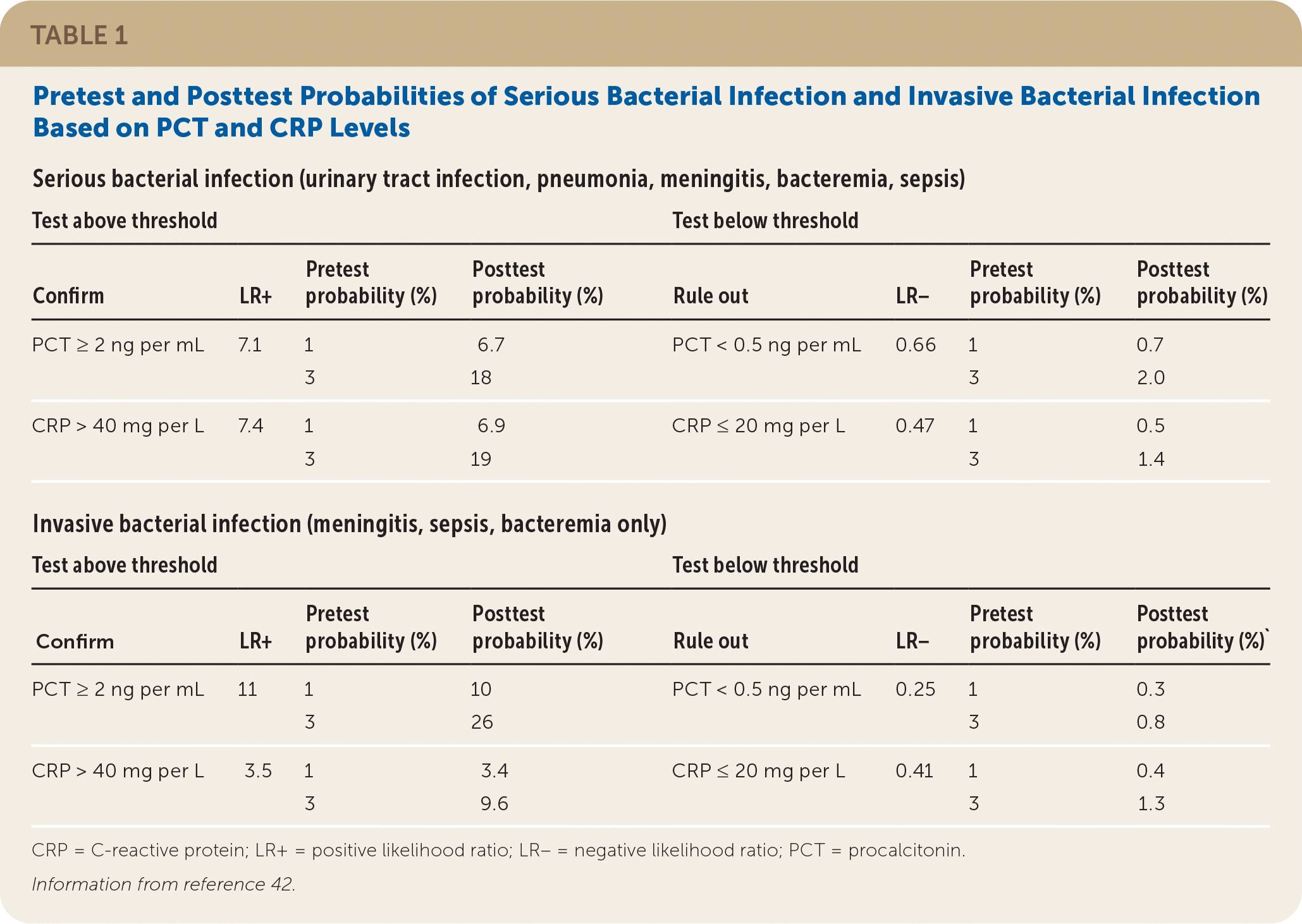
| Serious bacterial infection (urinary tract infection, pneumonia, meningitis, bacteremia, sepsis) | |||||||
| Test above threshold | Test below threshold | ||||||
| Confirm | LR+ | Pretest probability (%) | Posttest probability (%) | Rule out | LR− | Pretest probability (%) | Posttest probability (%) |
| PCT ≥ 2 ng per mL | 7.1 | 1 | 6.7 | PCT < 0.5 ng per mL | 0.66 | 1 | 0.7 |
| 3 | 18 | 3 | 2.0 | ||||
| CRP > 40 mg per L | 7.4 | 1 | 6.9 | CRP ≤ 20 mg per L | 0.47 | 1 | 0.5 |
| 3 | 19 | 3 | 1.4 | ||||
| Invasive bacterial infection (meningitis, sepsis, bacteremia only) | |||||||
| Test above threshold | Test below threshold | ||||||
| Confirm | LR+ | Pretest probability (%) | Posttest probability (%) | Rule out | LR− | Pretest probability (%) | Posttest probability (%)` |
| PCT ≥ 2 ng per mL | 11 | 1 | 10 | PCT < 0.5 ng per mL | 0.25 | 1 | 0.3 |
| 3 | 26 | 3 | 0.8 | ||||
| CRP > 40 mg per L | 3.5 | 1 | 3.4 | CRP ≤ 20 mg per L | 0.41 | 1 | 0.4 |
| 3 | 9.6 | 3 | 1.3 | ||||
LUMBAR PUNCTURE
Lumbar puncture for cerebrospinal fluid culture is recommended as part of the evaluation of febrile neonates one month and younger.11,46,47 The procedure is not recommended in infants older than three months unless neurologic signs (e.g., seizures or altered consciousness, including lethargy) are present.46,48
For infants between one and three months of age, there is a lack of consensus. A 2016 guideline from the American College of Emergency Physicians concluded that although no predictors adequately identify young infants for whom lumbar puncture is appropriate, it may still be considered.8 Suggestions for when lumbar puncture may be considered include toxic appearance or abnormal laboratory results, such as absolute neutrophil count greater than 10,000 per mm3 (10.0 × 109 per L), CRP greater than 20 mg per L, or PCT greater than 0.5 ng per mL.49
A retrospective study of infants up to 90 days of age in whom lumbar puncture was performed found that cerebrospinal fluid white blood cell count greater than 20 per mm3, protein greater than 100 mg per dL (1,000 mg per L), and glucose less than 20 mg per dL (1.11 mmol per L) each predicted positive cerebrospinal fluid culture, whereas elevated red blood cell count did not.50 The same series found that 81% of cerebrospinal fluid cultures positive for bacterial pathogens had detectable growth within 36 hours.50
IMAGING
Chest radiography is recommended for most children up to three years of age who exhibit signs of pneumonia, such as chest retractions, cough, hypoxia, or tachypnea.8,43,51 If a well-appearing child two months or older has wheezing suggestive of asthma or wheezing and fever suggestive of bronchiolitis rather than pneumonia, radiography may be omitted.8,52 For neonates younger than 28 days, chest radiography is often performed even in the absence of respiratory symptoms, but the benefits have not been proven.51
VIRAL TESTING
During influenza season, children younger than three years who present with fever and subsequently test positive for influenza have low rates of coincident serious bacterial infection.24,53 The Centers for Disease Control and Prevention cautions that nonmolecular rapid influenza tests have low sensitivity; if clinical suspicion is high, a negative test does not preclude a diagnosis of influenza.54 When COVID-19 is circulating, consider testing using a molecular assay.
A 2004 study suggested a reduced risk of serious bacterial infection in children younger than 60 days who tested positive for respiratory syncytial virus.55 However, a more recent study performed on febrile neonates 28 days and younger did not find any significant difference in the rate of serious bacterial infection based on respiratory syncytial virus status.16
Clinical Decision Algorithms
Several algorithms have been developed to help assess clinical risk for infants one to three months of age. The Step-by-Step17,43 and Laboratory Score26,56,57 were developed after the widespread use of pneumococcal conjugate vaccine and reflect the current risk of invasive pneumococcal disease; however, they require PCT and CRP testing. The older Rochester algorithm40 does not require these inflammatory markers. A comparison found that Step-by-Step had greater sensitivity for invasive bacterial infection; all three algorithms comparably identified low-risk children.17,43,58 Table 2 reviews these three algorithms,40,43,56 and online calculators are also available (Step-by-Step: https://www.mdcalc.com/step-step-approach-febrile-infants; Rochester: https://www.mdcalc.com/rochester-criteria-febrile-infants). Risk assessment models that do not assign high-risk status to every neonate younger than 21 days (with consequent recommendation for lumbar puncture) are being investigated.59
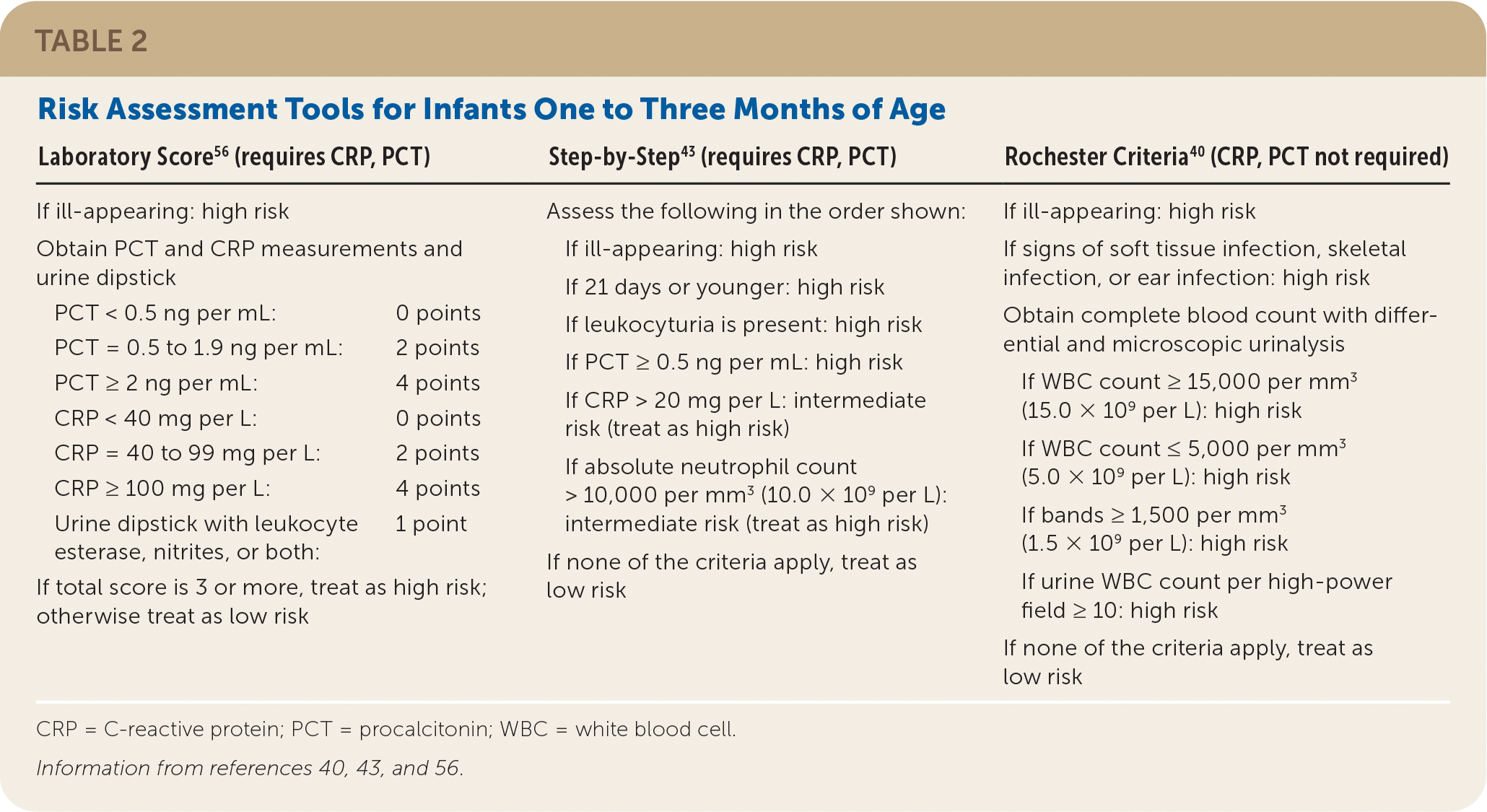
| Laboratory Score56 (requires CRP, PCT) | Step-by-Step43 (requires CRP, PCT) | Rochester Criteria40 (CRP, PCT not required) | |
|---|---|---|---|
| If ill-appearing: high risk | Assess the following in the order shown: If ill-appearing: high risk If 21 days or younger: high risk If leukocyturia is present: high risk If PCT ≥ 0.5 ng per mL: high risk If CRP > 20 mg per L: intermediate risk (treat as high risk) If absolute neutrophil count > 10,000 per mm3 (10.0 × 109 per L): intermediate risk (treat as high risk) If none of the criteria apply, treat as low risk | If ill-appearing: high risk If signs of soft tissue infection, skeletal infection, or ear infection: high risk Obtain complete blood count with differential and microscopic urinalysis If WBC count ≥ 15,000 per mm3 (15.0 × 109 per L): high risk If WBC count ≤ 5,000 per mm3 (5.0 × 109 per L): high risk If bands ≥ 1,500 per mm3 (1.5 × 109 per L): high risk If urine WBC count per high-power field ≥ 10: high risk If none of the criteria apply, treat as low risk | |
| Obtain PCT and CRP measurements and urine dipstick | |||
| PCT < 0.5 ng per mL: | 0 points | ||
| PCT = 0.5 to 1.9 ng per mL: | 2 points | ||
| PCT ≥ 2 ng per mL: | 4 points | ||
| CRP < 40 mg per L: | 0 points | ||
| CRP = 40 to 99 mg per L: | 2 points | ||
| CRP ≥ 100 mg per L: | 4 points | ||
| Urine dipstick with leukocyte esterase, nitrites, or both: | 1 point | ||
| If total score is 3 or more, treat as high risk; otherwise treat as low risk |
Management Strategies
Table 3 shows the management of unexplained fever in children 36 months and younger.8,11,18,24,37,42,43,45–47,51,53 In children being considered for inpatient management, empiric antibiotics should be initiated only after appropriate cultures have been obtained. The choice of treatment depends on local resistance patterns.11,19
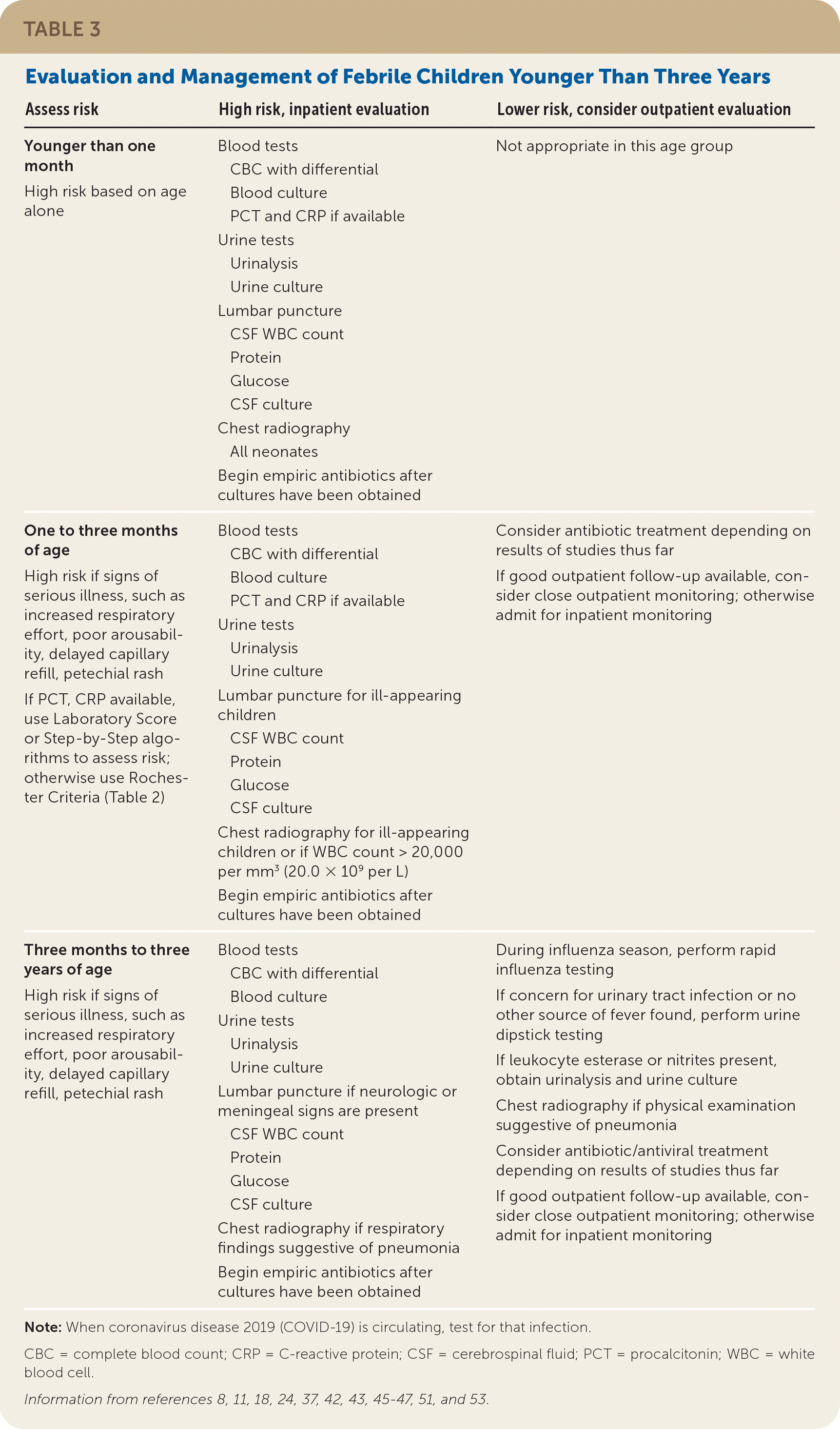
| Assess risk | High risk, inpatient evaluation | Lower risk, consider outpatient evaluation |
|---|---|---|
| Younger than one month High risk based on age alone | Blood tests CBC with differential Blood culture PCT and CRP if available Urine tests Urinalysis Urine culture Lumbar puncture CSF WBC count Protein Glucose CSF culture Chest radiography All neonates Begin empiric antibiotics after cultures have been obtained | Not appropriate in this age group |
| One to three months of age High risk if signs of serious illness, such as increased respiratory effort, poor arousability, delayed capillary refill, petechial rash If PCT, CRP available, use Laboratory Score or Step-by-Step algorithms to assess risk; otherwise use Rochester Criteria (Table 2) | Blood tests CBC with differential Blood culture PCT and CRP if available Urine tests Urinalysis Urine culture Lumbar puncture for ill-appearing children CSF WBC count Protein Glucose CSF culture Chest radiography for ill-appearing children or if WBC count > 20,000 per mm3 (20.0 × 109 per L) Begin empiric antibiotics after cultures have been obtained | Consider antibiotic treatment depending on results of studies thus far If good outpatient follow-up available, consider close outpatient monitoring; otherwise admit for inpatient monitoring |
| Three months to three years of age High risk if signs of serious illness, such as increased respiratory effort, poor arousability, delayed capillary refill, petechial rash | Blood tests CBC with differential Blood culture Urine tests Urinalysis Urine culture Lumbar puncture if neurologic or meningeal signs are present CSF WBC count Protein Glucose CSF culture Chest radiography if respiratory findings suggestive of pneumonia Begin empiric antibiotics after cultures have been obtained | During influenza season, perform rapid influenza testing If concern for urinary tract infection or no other source of fever found, perform urine dipstick testing If leukocyte esterase or nitrites present, obtain urinalysis and urine culture Chest radiography if physical examination suggestive of pneumonia Consider antibiotic/antiviral treatment depending on results of studies thus far If good outpatient follow-up available, consider close outpatient monitoring; otherwise admit for inpatient monitoring |
In neonates seven days or younger, coverage for Listeria is recommended. After the first week of life, the use of ampicillin for possible Listeria is controversial; rates of listeriosis are decreasing, and resistance is increasing.7 Recommended therapies in neonates include ampicillin combined with gentamicin,11 ampicillin combined with cefotaxime (Claforan),11,60 or cefotaxime alone.11 For young infants one to three months of age, there is no indication to use ampicillin to treat Listeria (uncommon) or Escherichia coli (frequent resistance). Appropriate antibiotics for this age group are ceftriaxone (Rocephin) or cefotaxime.11 The addition of vancomycin is recommended if meningitis caused by S. pneumoniae is suspected61 (Table 411,37,60–63).

| Age and findings | Therapy |
|---|---|
| Younger than one month | Ampicillin plus gentamicin Ampicillin,* 100 to 200 mg per kg per day IM or IV, divided, every six hours Gentamicin,† 2.5 mg per kg IM or IV every eight hours, with adjustments based on serum levels Alternative: Ampicillin plus cefotaxime (Claforan) Ampicillin,* 100 to 200 mg per kg per day IM or IV, divided, every six hours Cefotaxime,* 150 to 200 mg per kg per day IM or IV, divided, every six to eight hours Alternative: Cefotaxime alone, 150 to 200 mg per kg per day IM or IV, divided, every six to eight hours* If Streptococcus pneumoniae meningitis is suspected, add vancomycin, 20 mg per kg IV loading dose, then check creatinine levels: < 0.7 mg per dL, use 15 mg per kg every 12 hours 0.7 to 0.9, use 20 mg per kg every 24 hours 1.0 to 1.2, use 15 mg per kg every 24 hours 1.3 to 1.6, use 10 mg per kg every 24 hours > 1.6, use 15 mg per kg every 48 hours |
| One to three months, meningitis not suspected | Ceftriaxone (Rocephin), 50 to 75 mg per kg per day IM or IV, divided, every 12 to 24 hours Alternative: Cefotaxime, 75 to 200 mg per kg per day IM or IV, divided, every six to eight hours |
| One to three months, meningitis a concern | Ceftriaxone: Initial dose, 100 mg per kg IV; then 80 to 100 mg per kg per day IV, divided, every 12 to 24 hours; maximum dose, 4 g per 24 hours If S. pneumoniae is a concern, add vancomycin, 60 mg per kg per day IV, divided, every six hours |
| One to three months, urinary findings | Ceftriaxone, 75 mg per kg IV every 24 hours Alternative: Older than two months, oral cefixime (Suprax),‡ 8 mg per kg per day Alternative: Oral amoxicillin/clavulanate (Augmentin), 30 mg per kg per day (amoxicillin component), divided, every 12 hours |
| One to three months, suspected bacterial pneumonia, considering outpatient treatment | Oral amoxicillin, 90 mg per kg per day, divided, every 12 hours Alternative: Oral azithromycin (Zithromax), 10 mg per kg in single dose on day 1, then 5 mg per kg per day for days 2 to 5 |
| Older than three months, high risk, no localizing signs | Ceftriaxone, 50 to 75 mg per kg IV once daily Alternative: Cefotaxime, 150 to 180 mg per kg per day IM or IV, divided, every six to eight hours |
| Older than three months, suspected bacterial pneumonia | Amoxicillin, 90 mg per kg per day orally, divided, every 12 hours; maximum, 500 mg per dose Alternative: Oral azithromycin, 10 mg per kg in single dose on day 1, then 5 mg per kg orally for days 2 to 5 Severe infection: Ceftriaxone, 50 to 200 mg per kg per day IM or IV, divided, every 12 to 24 hours; maximum, 2 g per day |
| Older than three months, urinary findings | Oral cefixime,‡ 8 mg per kg per day Alternative: Oral amoxicillin/clavulanate, 20 to 40 mg per kg per day (amoxicillin component), divided, every eight hours Alternative: Trimethoprim/sulfamethoxazole, 8 to 12 mg per kg per day (trimethoprim component) orally or IV, divided, every six to 12 hours |
No empiric antibiotic treatment is needed for febrile older infants and children three to 36 months of age who have normal urinalysis and no localizing signs.11 Children with suspected pneumonia may be treated with amoxicillin or azithromycin (Zithromax)62; those with UTI can be treated with cefixime (Suprax), amoxicillin/clavulanate (Augmentin), or trimethoprim/sulfamethoxazole.37 The decision to hospitalize or discharge the patient should be based in part on the ability of the family to bring the patient back, if needed, for further evaluation.21,49
This article updates previous articles on this topic by Hamilton and John1 ; Sur and Bukont64 ; and Luszczak.65
Data Sources: A literature search was conducted with Medline/Ovid using search terms that included neonatal fever, childhood fever, bacterial illness children, bacterial infection children, urinary tract infection children, and thermometer accuracy. We also searched the Cochrane database, Essential Evidence Plus, and Web of Knowledge. Additionally, we identified articles in which the pre-Hib, pre-pneumococcal vaccine recommendations were originally published, as well as recent studies and reviews that cite these articles. Search dates: December 11 to 14, 2018; January 11 and 20, 2019; and April 20 to 22, 2020.
