Am Fam Physician. 2020;102(1):53-54
Author disclosure: No relevant financial affiliations.
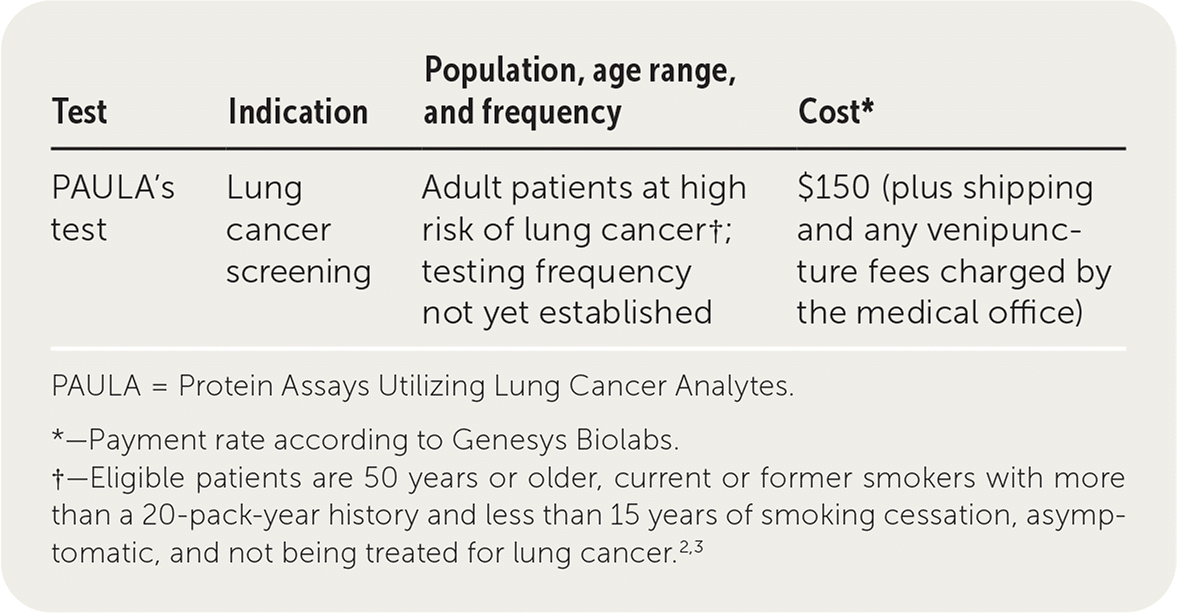
PAULA's test (Protein Assays Utilizing Lung Cancer Analytes) is a blood test for early detection of lung cancer in high-risk adults.1 Eligible patients are 50 years or older, current or former smokers with more than a 20-pack-year history and less than 15 years of smoking cessation, asymptomatic, and not being treated for lung cancer.2,3 The test is composed of a panel of three tumor markers (carcinoembryonic antigen, cancer antigen 125, and CYFRA 21-1 [a fragment of cytokeratin 19]) and one autoantibody marker (New York esophageal squamous cell carcinoma 1 [NY-ESO-1]).
Accuracy
A 2015 retrospective study used a training set of 115 patients with confirmed non–small cell lung carcinoma and 115 patients (matched in age and smoking history) in a control group to evaluate the predictive model used in PAULA's test.3 It was then validated in an independent set of 150 matched patients. This study found that the panel of four biomarkers was 77% sensitive and 80% specific for the detection of lung cancer in the validation group (positive likelihood ratio = 3.8; negative likelihood ratio = 0.29).3 Given a 1% likelihood of lung cancer in the target screening population, the positive predictive value is estimated to be 3.7%, and the negative predictive value is estimated to be 99.7%.
A 2018 study that included a training set of 268 patients with lung cancer and 336 patients in a control group, plus a validation set of 155 patients with lung cancer and 245 patients in a control group, explored adding a fifth biomarker (hepatocyte growth factor) to the established panel.4 Both the four- and five-biomarker panels had a sensitivity of 49% and a specificity of 96%.
The U.S. Preventive Services Task Force recommends annual screening for lung cancer with low-dose chest computed tomography (CT) in adults 55 to 80 years of age who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.5 The National Lung Screening Trial (NLST) randomized 53,439 asymptomatic participants, 55 to 74 years of age with at least a 30-pack-year smoking history, to annual screening with low-dose CT or chest radiography for three years. Low-dose CT had a sensitivity of 93.8% and specificity of 73.4% for lung cancer.6
Benefit
There have been no prospective studies demonstrating that PAULA's test improves patient-oriented outcomes.
Harms
PAULA's test combines tumor markers with an autoantibody marker to increase the overall sensitivity of the test, although it is still less sensitive than screening with low-dose CT. Preliminary testing of PAULA's test demonstrated increased expression of biomarkers in patients with benign pulmonary disease (e.g., chronic obstructive pulmonary disease, asthma, bronchitis), which could lead to false-positive findings.3 As a result, patients may undergo unnecessary and potentially invasive diagnostic testing, including full-dose CT and biopsy. The 2018 validation study also demonstrated lower sensitivity than previously documented, but the reasons for this are unclear.4
Cost
Bottom Line
Because of insufficient evidence of benefit, no recommendations can be made regarding PAULA's test. Prospective cohort studies of a screening population and randomized controlled trials comparing cancer mortality outcomes using PAULA's test vs. low-dose CT and no screening are needed before the test can be recommended as a screening option.