Am Fam Physician. 2020;102(1):5-6
Related letter: Beware of the Differing Definitions for the False-Positive Rate
Published online April 27, 2020.
Author disclosure: No relevant financial affiliations.
To the Editor: Antibody testing will become increasingly important as the coronavirus disease 2019 (COVID-19) pandemic progresses. In the setting of highly selected antigen testing, antibody tests will help public health officials determine the extent of previous infection, even among asymptomatic individuals and those with mild symptoms who did not seek medical care. Antibody testing is also likely to be part of the foundation for determining the pace of relaxing current physical distancing measures. Finally, clinicians will use antibody testing to counsel individual patients about whether they have recently had COVID-19 or to determine their immunity.
New rapid antibody tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, are qualitative. Analogous to home pregnancy tests, these antibody tests are positive or negative. By varying the cutoff that defines a positive test result for immunoglobulin G (IgG) or IgM, test developers can choose to favor a high sensitivity, a high specificity, or take a balanced approach. Cellex, the first antibody test approved by the U.S. Food and Drug Administration for the virus, has a reported sensitivity of 94% and specificity of 96%.1 However, as we begin widespread testing in a population in which the prevalence of previous SARS-CoV-2 infection is unknown, there is a risk of false-positive results. When initially diagnosing acute infection, it is important to avoid false-negatives because this can falsely reassure patients and hinder appropriate contact tracing and isolation. However, when assessing whether patients had a previous infection and may be immune, it is important to avoid false-positives so that patients do not think they are immune when they are not.
Table 1 summarizes the false-positive rates at various population prevalence for the Cellex test and for a hypothetical test that is 90% sensitive and 99% specific.1 At relatively low population prevalences, which likely reflect current conditions in the United States and elsewhere, we would argue that false-positive rates are unacceptably high with the Cellex test. Many of the other tests with provisional approval by the U.S. Food and Drug Administration have not been appropriately evaluated for accuracy.2
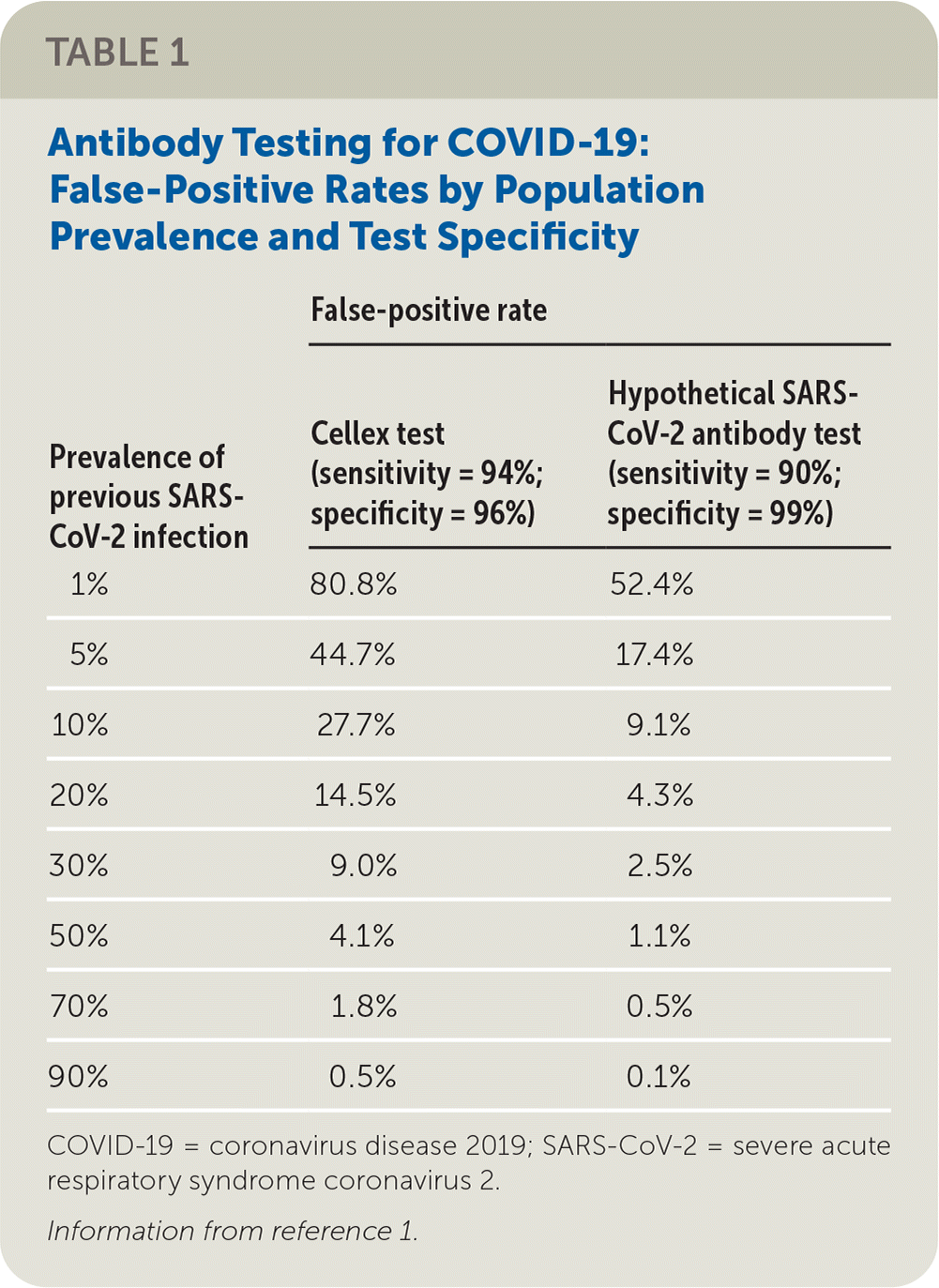
| Prevalence of previous SARS- CoV-2 infection | False-positive rate | |
|---|---|---|
| Cellex test (sensitivity = 94%; specificity = 96%) | Hypothetical SARS-CoV-2 antibody test (sensitivity = 90%; specificity = 99%) | |
| 1% | 80.8% | 52.4% |
| 5% | 44.7% | 17.4% |
| 10% | 27.7% | 9.1% |
| 20% | 14.5% | 4.3% |
| 30% | 9.0% | 2.5% |
| 50% | 4.1% | 1.1% |
| 70% | 1.8% | 0.5% |
| 90% | 0.5% | 0.1% |
Therefore, we encourage family physicians to look for appropriately validated antibody tests with adequate specificity (ideally 99% or higher), even if it means sacrificing some sensitivity. Also, they should encourage laboratories to report test results in a way that reflects the local population prevalence based on widespread testing and include the false-positive rate. This information is needed to help family physicians better inform shared decision-making regarding previous infection and return to work or school.
Editor's Note: Dr. Ebell is deputy editor for evidence-based medicine for AFP.