Am Fam Physician. 2020;102(2):105-109
Summary of Recommendations
The USPSTF recommends against screening for bacterial vaginosis in pregnant persons who are not at increased risk for preterm delivery (Table 1). D recommendation.
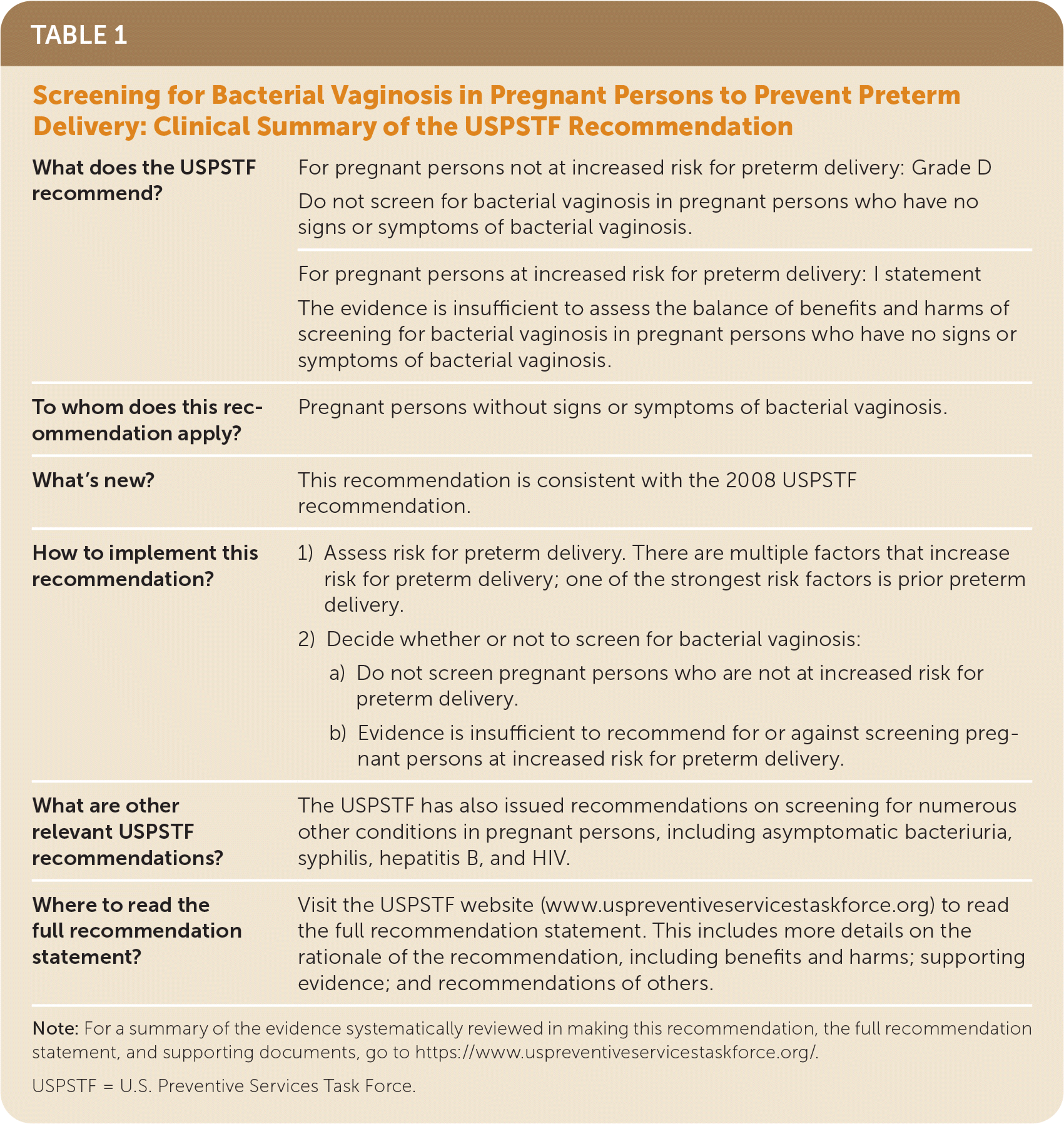
| What does the USPSTF recommend? | For pregnant persons not at increased risk for preterm delivery: Grade D Do not screen for bacterial vaginosis in pregnant persons who have no signs or symptoms of bacterial vaginosis. |
| For pregnant persons at increased risk for preterm delivery: I statement The evidence is insufficient to assess the balance of benefits and harms of screening for bacterial vaginosis in pregnant persons who have no signs or symptoms of bacterial vaginosis. | |
| To whom does this recommendation apply? | Pregnant persons without signs or symptoms of bacterial vaginosis. |
| What's new? | This recommendation is consistent with the 2008 USPSTF recommendation. |
| How to implement this recommendation? | 1) Assess risk for preterm delivery. There are multiple factors that increase risk for preterm delivery; one of the strongest risk factors is prior preterm delivery. 2) Decide whether or not to screen for bacterial vaginosis: a) Do not screen pregnant persons who are not at increased risk for preterm delivery. b) Evidence is insufficient to recommend for or against screening pregnant persons at increased risk for preterm delivery. |
| What are other relevant USPSTF recommendations? | The USPSTF has also issued recommendations on screening for numerous other conditions in pregnant persons, including asymptomatic bacteriuria, syphilis, hepatitis B, and HIV. |
| Where to read the full recommendation statement? | Visit the USPSTF website (www.uspreventiveservicestaskforce.org) to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for bacterial vaginosis in pregnant persons who are at increased risk for preterm delivery (Table 1). I recommendation.
See the Practice Considerations section for more information on risk assessment and suggestions for practice regarding the I statement.
Importance
Bacterial vaginosis is common and is caused by a disruption of the microbiological environment in the lower genital tract. In the United States, reported prevalence of bacterial vaginosis among pregnant women ranges from 5.8% to 19.3% and is higher in some races/ethnicities.1 Bacterial vaginosis during pregnancy has been associated with adverse obstetrical outcomes including preterm delivery,2 early miscarriage,3 postpartum endometritis,4 and low birth weight.5 Bacterial vaginosis is often asymptomatic, can resolve spontaneously, and recurs often, with or without treatment.6 Most clinicians treat symptomatic bacterial vaginosis in pregnancy. The current recommendation statement focuses on screening for asymptomatic bacterial vaginosis in pregnancy.
In the United States, approximately 10% of live births are preterm (born prior to 37 weeks' gestation).7 Preterm birth is associated with serious complications, including major intraventricular hemorrhage, acute respiratory illnesses, and sepsis.7–10 Approximately two-thirds of all infant deaths in the United States occur among infants born preterm.8 The frequency and severity of adverse outcomes from preterm delivery are higher with earlier gestational age.
Assessment of Magnitude of Net Benefit
The USPSTF concludes with moderate certainty that screening for asymptomatic bacterial vaginosis in pregnant persons not at increased risk for preterm delivery has no net benefit in preventing preterm delivery.
The USPSTF concludes that for pregnant persons at increased risk for preterm delivery, the evidence is insufficient and conflicting, and the balance of benefits and harms cannot be determined.
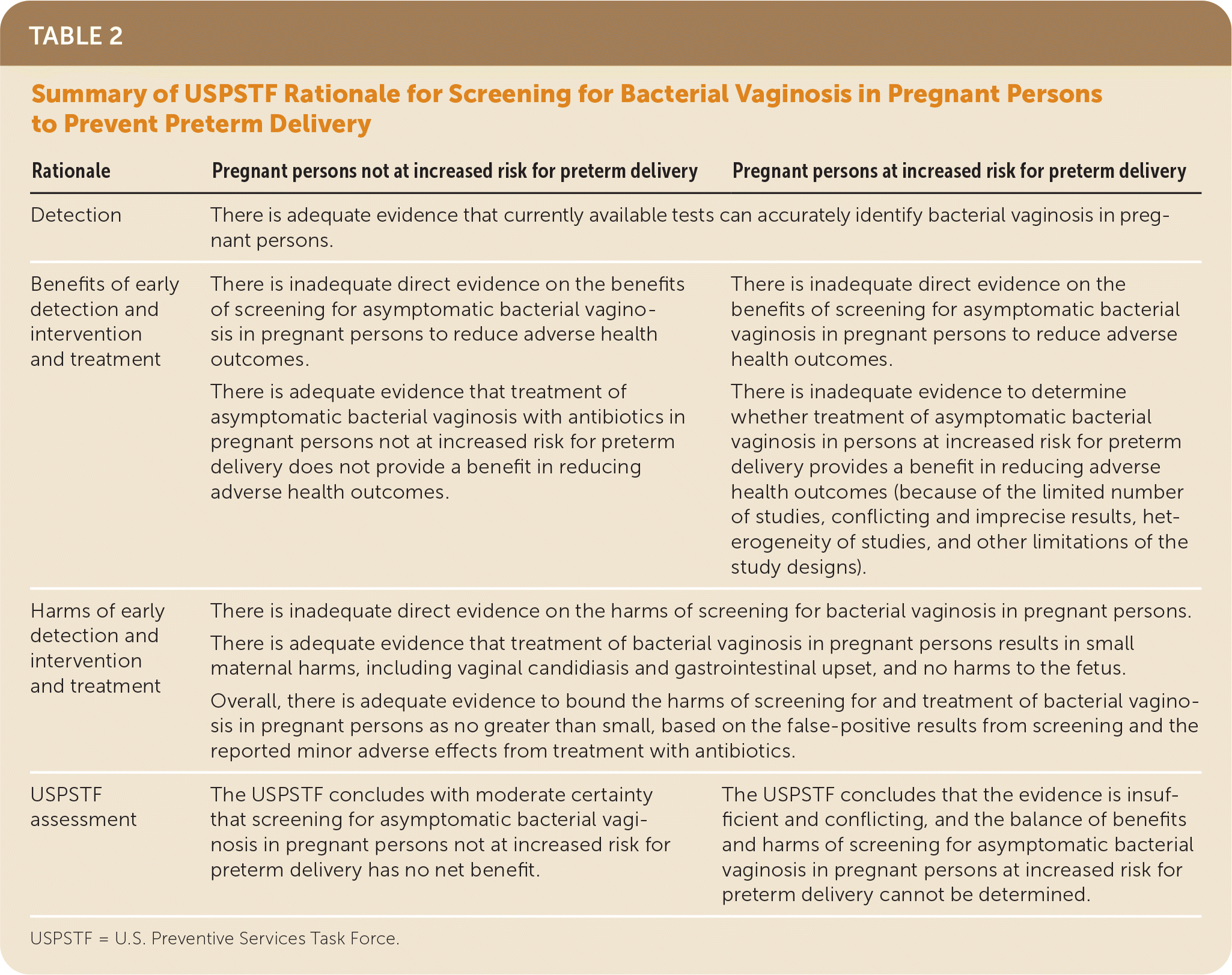
| Rationale | Pregnant persons not at increased risk for preterm delivery | Pregnant persons at increased risk for preterm delivery |
|---|---|---|
| Detection | There is adequate evidence that currently available tests can accurately identify bacterial vaginosis in pregnant persons. | |
| Benefits of early detection and intervention and treatment | There is inadequate direct evidence on the benefits of screening for asymptomatic bacterial vaginosis in pregnant persons to reduce adverse health outcomes. There is adequate evidence that treatment of asymptomatic bacterial vaginosis with antibiotics in pregnant persons not at increased risk for preterm delivery does not provide a benefit in reducing adverse health outcomes. | There is inadequate direct evidence on the benefits of screening for asymptomatic bacterial vaginosis in pregnant persons to reduce adverse health outcomes. There is inadequate evidence to determine whether treatment of asymptomatic bacterial vaginosis in persons at increased risk for preterm delivery provides a benefit in reducing adverse health outcomes (because of the limited number of studies, conflicting and imprecise results, heterogeneity of studies, and other limitations of the study designs). |
| Harms of early detection and intervention and treatment | There is inadequate direct evidence on the harms of screening for bacterial vaginosis in pregnant persons. There is adequate evidence that treatment of bacterial vaginosis in pregnant persons results in small maternal harms, including vaginal candidiasis and gastrointestinal upset, and no harms to the fetus. Overall, there is adequate evidence to bound the harms of screening for and treatment of bacterial vaginosis in pregnant persons as no greater than small, based on the false-positive results from screening and the reported minor adverse effects from treatment with antibiotics. | |
| USPSTF assessment | The USPSTF concludes with moderate certainty that screening for asymptomatic bacterial vaginosis in pregnant persons not at increased risk for preterm delivery has no net benefit. | The USPSTF concludes that the evidence is insufficient and conflicting, and the balance of benefits and harms of screening for asymptomatic bacterial vaginosis in pregnant persons at increased risk for preterm delivery cannot be determined. |
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation statement applies to pregnant persons without symptoms of bacterial vaginosis.
DEFINITION
Healthy vaginal flora comprises more than 90% lactobacilli. Bacterial vaginosis occurs when there is a shift in this flora to include a greater proportion of mixed anaerobic bacteria, such as the Gardnerella, Prevotella, and Atopobium species.12,13 Most often, bacterial vaginosis is asymptomatic. When symptoms occur, they include off-white, thin, homogenous discharge, a vaginal “fishy” odor, or both.
ASSESSMENT OF RISK
Persons who are not at increased risk for preterm delivery include pregnant persons with no history of previous preterm delivery or other risk factors for preterm delivery. Whereas multiple factors increase risk for preterm delivery, one of the strongest risk factors is prior preterm delivery.
See the Potential Preventable Burden section for additional information on risk factors for preterm delivery.
SCREENING TESTS
Screening tests for bacterial vaginosis are performed on vaginal secretions obtained during a pelvic examination in a primary care setting. Available screening tests include nucleic acid assays, sialidase assays, and clinical assessment (i.e., using the Amsel criteria of pH, vaginal discharge, clue cells, and “whiff test”).
TREATMENT
Oral metronidazole and oral clindamycin, as well as vaginal metronidazole gel or clindamycin cream, are the usual treatments for symptomatic bacterial vaginosis. The optimal treatment regimen for pregnant persons with bacterial vaginosis is unclear.
ADDITIONAL TOOLS AND RESOURCES
The Centers for Disease Control and Prevention website provides current treatment recommendations.14
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENT
Potential Preventable Burden. Bacterial vaginosis occurs in as many as 29% of women in the United States15 and in 5.8% to 19.3% of pregnant women, depending on the specific population being studied.1,16 Reported factors that increase the likelihood of a diagnosis of bacterial vaginosis include African American race, poverty, smoking, increased body mass index, vaginal douching, low educational attainment, and certain sexual behaviors, including a high number of partners, lack of condom or contraceptive use, vaginal sex, sex with a female partner, and concurrent sexually transmitted infections.6,15,17,18
Causes of preterm delivery are likely multifactorial, and numerous risk factors are associated with an increased risk for preterm birth.6 History of a prior preterm delivery is associated with 2.5-fold higher odds for preterm delivery in subsequent pregnancies.19 Whereas bacterial vaginosis during pregnancy is associated with twofold higher odds for preterm delivery,2 it is not clear that bacterial vaginosis is a cause of preterm delivery. Other additional risk factors for preterm delivery include, but are not limited to, cervical insufficiency, multifetal gestation, young or advanced maternal age, low maternal body mass index (< 20, calculated as weight in kilograms divided by height in meters squared), genitourinary infections, HIV infection, and other maternal medical conditions.6,20–23 The association of these additional risk factors with preterm delivery is small to moderate, and factors can act in isolation or in combination. Preterm birth rates also vary by race/ethnicity in the United States; recent data report preterm birth rates of 8.6% among Asian women, 11.8% among Native Hawaiian/Other Pacific Islander women, 9.7% among Hispanic women, 11.5% among American Indian/Alaska Native women, 14.1% among black women, and 9.1% among white women.7 Among women with a prior preterm delivery, the rate of recurrent preterm delivery in African American women is 4 times higher than the rate of recurrent preterm delivery in white women.20 Even when these risk factors are present, it is unclear whether screening and treating asymptomatic bacterial vaginosis in pregnant persons at increased risk for preterm delivery prevents preterm delivery.
African American race is both associated with bacterial vaginosis and strongly associated with preterm delivery. Other factors associated with both bacterial vaginosis and preterm delivery include young age, nulliparity, current tobacco use, low educational attainment, lower income, and concurrent sexually transmitted infections.
Five studies provided evidence on the benefit of treatment of bacterial vaginosis in women with a previous preterm delivery for reducing the incidence of preterm delivery. Four of these studies evaluated the treatment of bacterial vaginosis with oral metronidazole6 and reported the incidence of preterm delivery at less than 37 weeks. Three of these studies reported statistically significant absolute reductions in preterm delivery after treatment (ranging from 18% to 29% absolute reductions in risk), and 1 study reported no significant difference. Limitations of the evidence, including imprecision, the fact that some of the results were from subgroup analyses, and the inconsistency of results, prevented a definitive conclusion about the benefit.6 Two studies (1 evaluating oral metronidazole and the other evaluating vaginal clindamycin) presented results for preterm delivery at less than 34 weeks, and the results were mixed.6
Potential Harms. The harms of screening for bacterial vaginosis in pregnant persons and treatment with antibiotics generally involve adverse effects such as gastrointestinal upset and vaginal candidiasis.6 Four observational studies and 2 large meta-analyses of observational studies on the use of metronidazole during pregnancy for any reason (not limited to bacterial vaginosis) reported no increase in congenital malformations or incident cancer in children exposed in utero.24–29
Current Practice. No data are available on how frequently pregnant persons at increased risk for preterm delivery are screened for bacterial vaginosis during pregnancy, but screening in asymptomatic pregnant persons is not recommended by any large U.S. professional organization. Clinicians routinely test and treat pregnant persons for symptomatic bacterial vaginosis.
Other Related USPSTF Recommendations
This recommendation statement was first published in JAMA. 2020;323(13):1286–1292.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” “Research Needs and Gaps,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/bacterial-vaginosis-in-pregnancy-to-prevent-preterm-delivery-screening.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.