This is a corrected version of the article that appeared in print.
Am Fam Physician. 2020;102(2):121-124
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Routine blood cultures, sputum cultures, and urinary antigen testing are not beneficial in patients with nonsevere CAP.
• The Pneumonia Severity Index is recommended to determine the need for hospitalization, whereas the ATS/IDSA criteria for severe CAP are recommended to predict the need for intensive care.
• Routine treatment of CAP with macrolide monotherapy is no longer recommended unless local resistance is low. Amoxicillin and doxycycline are preferred in low-risk patients.
• Five-day treatment courses are recommended for all patients with CAP, with reassessment following treatment.
From the AFP Editors
The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) recently updated their recommendations on the diagnosis and treatment of community-acquired pneumonia (CAP). This guideline focuses on immunocompetent U.S. adults who have not recently traveled internationally, particularly to regions with emerging respiratory pathogens. It predates the coronavirus disease 2019 (COVID-19) pandemic.
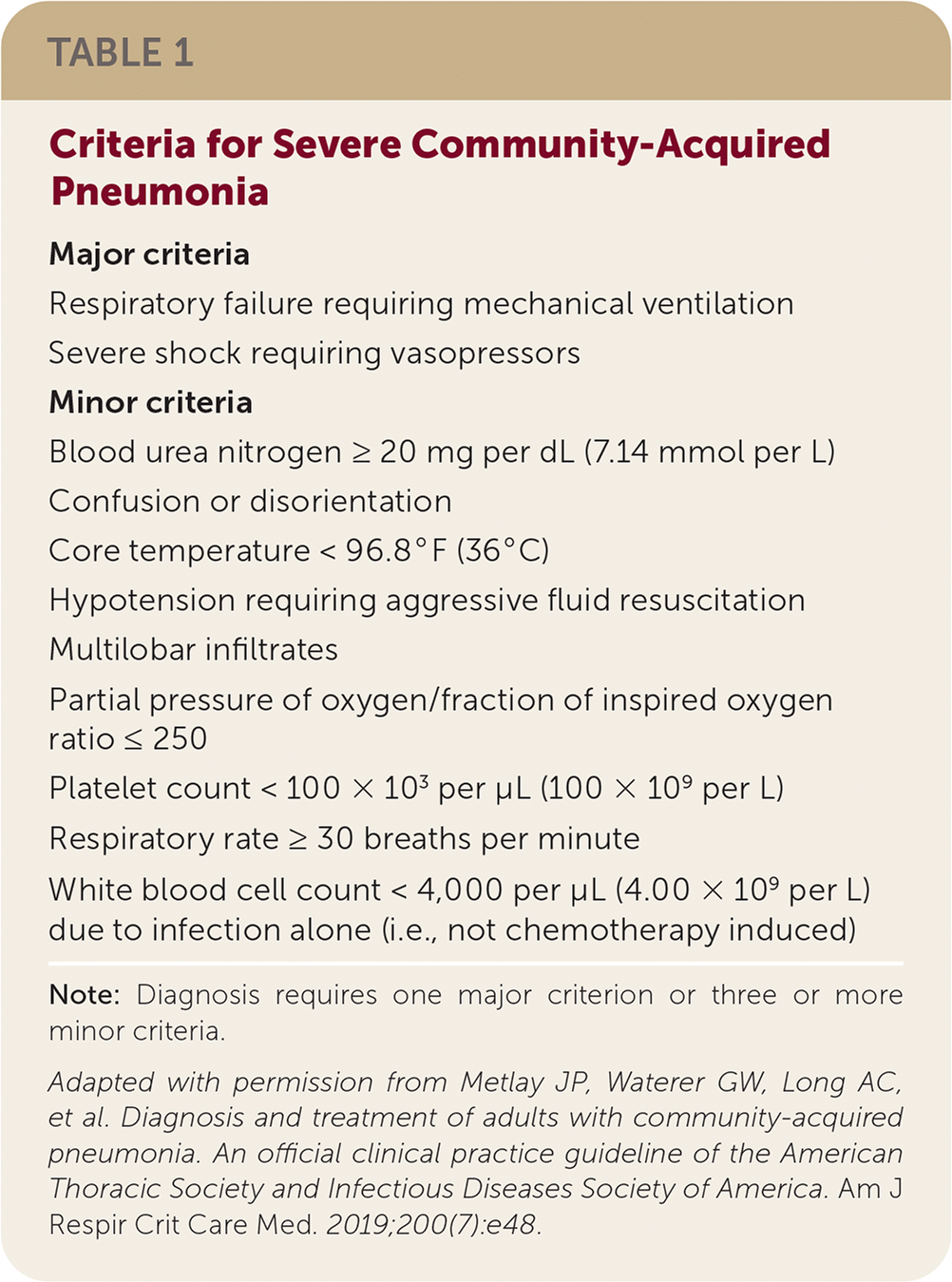
The ATS/IDSA guideline replaces grading of CAP by care setting (e.g., outpatient, inpatient general care, intensive care unit [ICU]) because decisions about the site of care can vary widely. Instead, the updated recommendations are based on validated illness severity criteria, as shown in Table 1. Severe CAP is defined as the presence of one major criterion or at least three minor criteria.
| Major criteria Respiratory failure requiring mechanical ventilation Severe shock requiring vasopressors Minor criteria Blood urea nitrogen ≥ 20 mg per dL (7.14 mmol per L) Confusion or disorientation Core temperature < 96.8°F (36°C) Hypotension requiring aggressive fluid resuscitation Multilobar infiltrates Partial pressure of oxygen/fraction of inspired oxygen ratio ≤ 250 Platelet count < 100 × 103 per μL (100 × 109 per L) Respiratory rate ≥ 30 breaths per minute White blood cell count < 4,000 per μL (4.00 × 109 per L) due to infection alone (i.e., not chemotherapy induced) |
Diagnostic Testing
BLOOD AND SPUTUM CULTURES
Routine blood and sputum cultures have not been shown to improve patient outcomes in CAP. Sputum cultures are recommended before treatment initiation only if the patient has severe CAP, and particularly if he or she is intubated; if the patient has a history of methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa infection or if he or she is being empirically treated for these pathogens; or if the patient was hospitalized and received parenteral antibiotics within the previous 90 days. Lower respiratory tract samples from intubated patients with severe CAP should be sent for Gram stain and culture soon after intubation because of the higher risk of MRSA or P. aeruginosa infection, and because endotracheal aspirates have a higher yield than sputum samples. Blood cultures should be obtained if the patient has severe CAP; was previously or is currently being treated empirically for MRSA or P. aeruginosa infection, particularly respiratory infections; or if the patient was hospitalized and received parenteral antibiotics within the previous 90 days. The yield of blood cultures is low (2% to 9%) in adults without severe CAP, and empiric therapy is rarely changed based on these results.
URINARY ANTIGEN TESTING
Urinary antigen testing for Legionella or pneumococcus has not been shown to improve outcomes. Based on low-quality evidence, the ATS/IDSA guideline suggests that routine urine testing for pneumococcal antigen be performed only in severe cases of CAP and that urine testing for Legionella antigen be performed only in cases of severe CAP, exposure to a Legionella outbreak, or travel to an endemic area.
INFLUENZA TESTING
Although no studies have evaluated the impact of influenza testing on outcomes in adults with CAP, the importance of testing in the general population is well established. When influenza viruses are circulating in the community, influenza testing should be performed in all patients with CAP. A rapid influenza nucleic acid amplification test is preferred over a rapid influenza antigen test.
Treatment
OUTPATIENT TREATMENT VS. HOSPITALIZATION
In addition to clinical judgment, clinicians should use a validated clinical prediction rule to determine the need for hospitalization in patients with CAP. Based on low-quality evidence, the Pneumonia Severity Index (PSI) is preferred over the CURB-65 (confusion, urea level, respiratory rate, blood pressure, and age 65 or older). Although the PSI can underestimate the severity of illness among younger patients, it has higher discriminative power in predicting mortality and a lower false-positive rate than the CURB-65.
Although the PSI and CURB-65 estimate mortality risk, they do not effectively determine the level of care required. Patients with hypotension requiring vasopressors or respiratory failure requiring mechanical ventilation need ICU-level care. For other patients, the criteria for severe CAP can augment clinical judgment in determining the need for higher-intensity treatment. The ATS/IDSA criteria for severe CAP predict ICU admission with a sensitivity of 84% and a specificity of 78%. Without major criteria, the presence of three or more minor criteria for severe CAP predicts ICU admission with a sensitivity of 56% and a specificity of 91%.
ANTIBIOTIC THERAPY
Several studies have evaluated whether the serum procalcitonin level can be used to distinguish bacterial pneumonia from viral infections. Because its sensitivity to detect bacterial infection ranges from 38% to 91%, the procalcitonin level is not accurate enough to justify withholding antibiotics in patients with suspected CAP.
Table 2 lists options for antibiotic regimens for the empiric treatment of CAP in the outpatient setting. [corrected] Randomized controlled trials have shown little evidence that one regimen is superior to others. Antibiotic choice depends on individualized risk-benefit analysis, weighing local antibiotic sensitivity data against patient characteristics such as drug allergies, the risk of cardiac arrhythmia (with macrolide antibiotics) or vascular disease (with fluoroquinolones), or history of Clostridioides difficile (formerly Clostridium difficile) infection. Despite concerns about adverse effects associated with fluoroquinolones, the ATA/IDSA panel decided that these drugs are justified for the treatment of outpatients with comorbidities and CAP.

| Patient factors | Antibiotic regimen* |
|---|---|
| No risk factors for methicillin-resistant Staphylococcus aureus or Pseudomonas aeruginosa† and No comorbidities listed below | Amoxicillin, 1,000 mg every 8 hours or Doxycycline, 100 mg every 12 hours or Macrolide if local pneumococcal resistance < 25%: Azithromycin (Zithromax), 500 mg on day 1, then 250 mg per day or Clarithromycin (Biaxin), 500 mg every 12 hours or Extended-release clarithromycin (Biaxin XL), 1,000 mg per day |
| Comorbidities: alcoholism; asplenia; diabetes mellitus; malignancy; or chronic heart, lung, liver, or kidney disease | Combination of: Amoxicillin/clavulanate (Augmentin), 500 mg/125 mg every 8 hours, 875 mg/125 mg every 12 hours, or 2,000 mg/125 mg every 12 hours or Cefpodoxime, 200 mg every 12 hours or Cefuroxime axetil, 500 mg every 12 hours PLUS Azithromycin, 500 mg on day 1, then 250 mg per day or Clarithromycin, 500 mg every 12 hours or Doxycycline, 100 mg every 12 hours or Extended-release clarithromycin, 1,000 mg per day OR Monotherapy with a respiratory fluoroquinolone: Gemifloxacin (Factive), 320 mg per day or Levofloxacin (Levaquin), 750 mg per day or Moxifloxacin (Avelox), 400 mg per day |
The ATS/IDSA panel no longer recommends the routine use of a macrolide antibiotic as monotherapy for CAP. This change was based on studies showing treatment failures with macrolides in patients with macrolide-resistant Streptococcus pneumoniae, in addition to macrolide resistance rates of more than 30% among S. pneumoniae isolates in the United States.
Table 3 lists options for antibiotic regimens for the empiric treatment of CAP in the inpatient setting. Omadacycline (Nuzyra), a newer tetracycline antibiotic, was shown to be as effective as moxifloxacin (Avelox) as monotherapy in adults with nonsevere CAP. However, because the safety of omadacycline is less established, the ATS/IDSA panel did not include it as a recommended treatment.

| Patient factors | Antibiotic regimen* | Patient factors | Antibiotic regimen* |
|---|---|---|---|
| Nonsevere pneumonia without risk factors for MRSA or Pseudomonas aeruginosa† | Combination of: Ampicillin/sulbactam (Unasyn), 1.5 to 3 g IV every 6 hours or Cefotaxime (Claforan), 1 to 2 g IV every 8 hours or Ceftaroline (Teflaro), 600 mg IV every 12 hours or Ceftriaxone (Rocephin), 1 to 2 g IV every day PLUS Azithromycin (Zithromax), 500 mg orally or IV every day or Clarithromycin (Biaxin), 500 mg orally every 12 hours OR Monotherapy with a respiratory fluoroquinolone: Levofloxacin (Levaquin), 750 mg orally or IV every day or Moxifloxacin (Avelox), 400 mg orally or IV every day | Severe pneumonia without risk factors for MRSA or P. aeruginosa† | Combination of: Ampicillin/sulbactam, 1.5 to 3 g IV every 6 hours or Cefotaxime, 1 to 2 g IV every 8 hours or Ceftaroline, 600 mg IV every 12 hours or Ceftriaxone, 1 to 2 g IV every day PLUS Azithromycin, 500 mg orally or IV every day or Clarithromycin, 500 mg orally every 12 hours or Levofloxacin, 750 mg orally or IV every day or Moxifloxacin, 400 mg orally or IV every day |
| Nonsevere pneumonia without risk factors for MRSA or P. aeruginosa† and Contraindications to macrolides and fluoroquinolones | Combination of: Ampicillin/sulbactam, 1.5 to 3 g IV every 6 hours or Cefotaxime, 1 to 2 g IV every 8 hours or Ceftaroline, 600 mg IV every 12 hours or Ceftriaxone, 1 to 2 g IV every day PLUS Doxycycline, 100 mg orally or IV every 12 hours | Severe pneumonia with locally validated risk factors for MRSA or P. aeruginosa† | For MRSA risk factors: Linezolid (Zyvox), 600 mg orally or IV every 12 hours or Vancomycin, 15 mg per kg IV every 12 hours (adjust based on levels) For P. aeruginosa risk factors: Aztreonam (Azactam), 2 g IV every 8 hours or Cefepime, 2 g IV every 8 hours or Ceftazidime (Fortaz), 2 g IV every 8 hours or Imipenem/cilastatin (Primaxin), 500 mg IV every 6 hours or Meropenem (Merrem IV), 1 g IV every 8 hours or Piperacillin/tazobactam (Zosyn), 4.5 g IV every 6 hours |
In inpatient and outpatient settings, five-day antibiotic courses are recommended, after which the patient should be verified as clinically stable with normal vital signs, normal mentation, and no difficulties with eating.
The category of health care–associated pneumonia (HCAP) has been eliminated because it does not predict antibiotic resistance. Empiric coverage for MRSA or P. aeruginosa is recommended only if locally validated risk factors are present, such as prior isolation of MRSA or P. aeruginosa from the respiratory tract or repeat hospitalization and treatment with parenteral antibiotics.
The ATS/IDSA panel considered whether suspected aspiration should be treated in patients with CAP but found no evidence to guide treatment. Aspiration is common: as many as one-half of adults aspirate during sleep. Adding anaerobic coverage for CAP is not recommended unless lung abscess or empyema is suspected.
CORTICOSTEROID AND ANTIVIRAL THERAPY
Corticosteroids should not be routinely prescribed for patients with CAP or severe influenzal pneumonia. Although two studies showed significant improvements in outcomes when corticosteroids were prescribed for CAP, these results are questionable because of study flaws, and subsequent studies have not shown improvement in clinically relevant outcomes.
Antiviral agents should be prescribed for adults with CAP who test positive for influenza, regardless of the duration of illness before diagnosis or the treatment setting. Although the benefits are greatest when antivirals are started within 48 hours of symptom onset, there is evidence supporting later initiation of therapy.
Editor's Note: This ATS/IDSA guideline was produced before the COVID-19 pandemic, which has altered the diagnosis and management of lower respiratory tract infections. The current version of this guideline limits testing and antiviral treatment in patients with influenza, and we expect testing for and treatment of severe acute respiratory syndrome coronavirus 2 infection to become a long-standing element of standard pneumonia care. Although the IDSA has published initial guidelines for management of COVID-19, the situation is evolving rapidly (https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management). Free information on COVID-19 is available from American Family Physician at https://www.aafp.org/journals/afp/explore/COVID-19.html and Essential Evidence Plus at http://www.essentialevidenceplus.com/content/eee/904.—Michael J. Arnold, MD, contributing editor
Guideline source: American Thoracic Society and Infectious Diseases Society of America
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Am J Respir Crit Care Med. October 1, 2019;200(7):e45–e67
Available at: https://bit.ly/2VnizAY