
Am Fam Physician. 2020;102(3):188-189
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Universal outpatient prophylactic anticoagulation of patients with cancer is not recommended.
• Patients with cancer who are at high risk of VTE benefit from low-dose anticoagulation during chemotherapy.
• Anticoagulation should be continued at least six months after VTE in patients with cancer. Continuing anticoagulation for 12 months reduces VTE recurrence similar to the increase in major bleeding.
From the AFP Editors
Patients with cancer experience more anticoagulant bleeding complications, making prevention and treatment decisions challenging. The American Society of Clinical Oncology has updated recommendations for the prevention and treatment of venous thromboembolism (VTE) in patients with cancer.
Universal VTE Prevention in Cancer
Routine anticoagulation for outpatients who have cancer without VTE is not recommended because it has not yielded improved survival. Risk factors such as cancer site or biomarker levels are unhelpful in identifying increased risk in ambulatory patients.
VTE Prevention During Chemotherapy
Systemic chemotherapy increases the risk of VTE. Prophylactic therapy with apixaban (Eliquis), rivaroxaban (Xarelto), or low-molecular-weight heparin (LMWH) should be considered for high-risk outpatients with cancer, providing there are no significant risk factors for bleeding and no drug interactions. LMWH has been shown to reduce the VTE rate by about one-half, but with more clinically relevant bleeding and unchanged mortality. Two recent trials showed low-dose direct oral anticoagulant (DOAC) treatment reduced symptomatic VTE in patients with cancer at higher risk of VTE. In one trial, apixaban at 2.5 mg twice daily prevented VTE with a number needed to treat of 17 and number needed to harm of 59 for major bleeding. In these studies, higher VTE risk was defined as a Khorana risk score of 2 or more (Table 1).
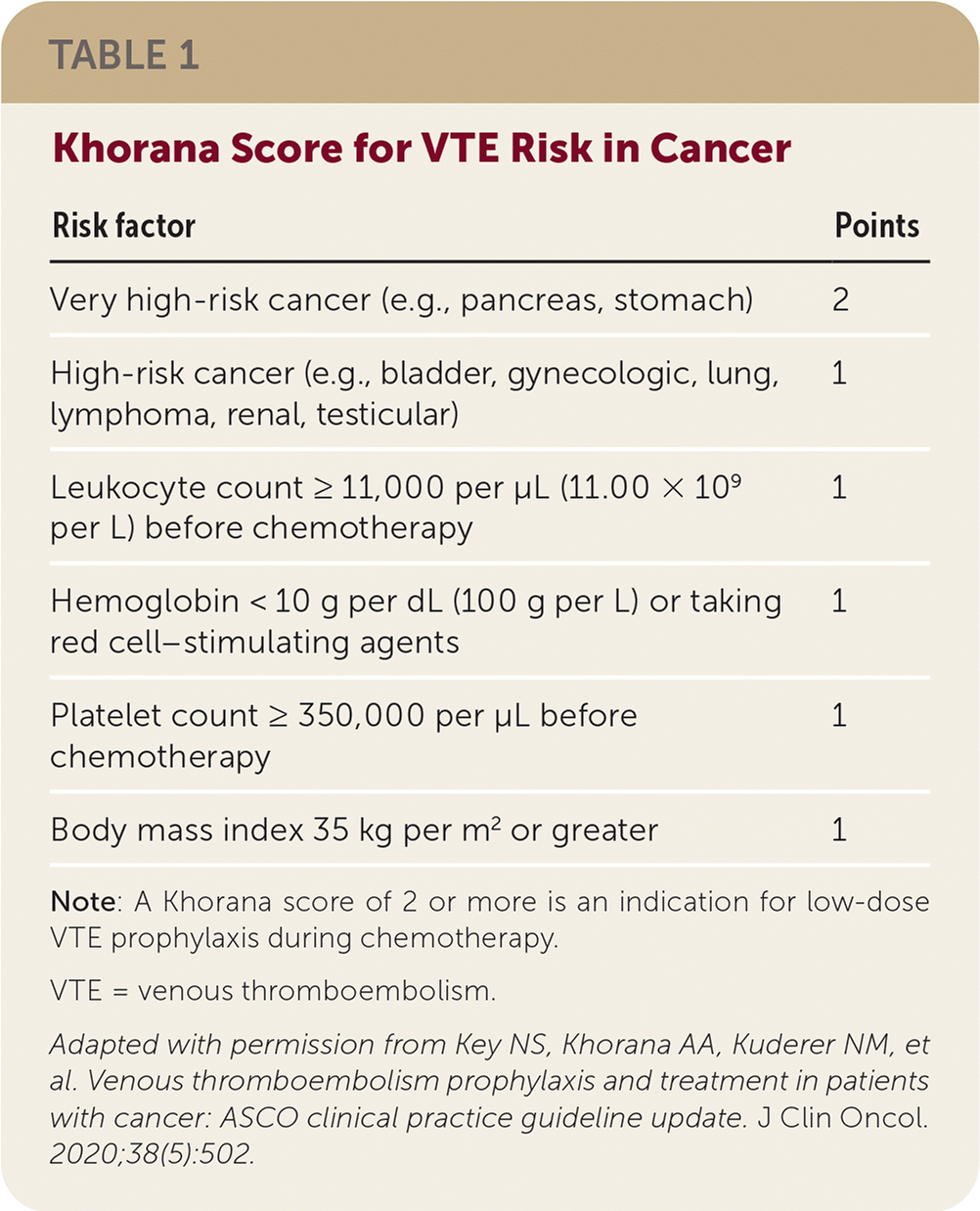
| Risk factor | Points |
|---|---|
| Very high-risk cancer (e.g., pancreas, stomach) | 2 |
| High-risk cancer (e.g., bladder, gynecologic, lung, lymphoma, renal, testicular) | 1 |
| Leukocyte count ≥ 11,000 per μL (11.00 × 109 per L) before chemotherapy | 1 |
| Hemoglobin < 10 g per dL (100 g per L) or taking red cell–stimulating agents | 1 |
| Platelet count ≥ 350,000 per μL before chemotherapy | 1 |
| Body mass index 35 kg per m2 or greater | 1 |
Although not included in the Khorana model, multiple myeloma is a high-risk cancer, especially when treated with thalidomide-based chemotherapy. Prophylaxis with LMWH is recommended for these patients.
VTE Prevention During Hospital Admission
Hospital admission increases VTE risk, and pharmacologic prophylaxis is recommended unless the bleeding risk is high.
SURGERY
Patients with cancer have elevated VTE risk for several weeks after surgery. All patients with cancer should start unfractionated heparin or LMWH prophylaxis before surgery and continue treatment after surgery. Mechanical prophylaxis is recommended as an adjunct to heparin therapy, but it is insufficient as monotherapy unless bleeding risk is high. Unfractionated heparin or LMWH prophylaxis is recommended for at least seven to 10 days after major cancer surgery. Patients at especially high risk for VTE because of restricted mobility, obesity, or previous VTE should receive an additional four weeks of LMWH prophylaxis after abdominal or pelvic cancer surgery. A recent study of laparoscopic and open surgeries demonstrated similar extended prophylaxis benefits.
MEDICAL ADMISSION
Although evidence is lacking, pharmacologic prophylaxis is recommended for patients with cancer and low bleeding risk based on evidence from other high-risk VTE populations. Although medical hospitalization increases risk, evidence in patients with cancer is limited because of a lack of studies of VTE prophylaxis in these patients. Prophylaxis is not recommended for patients with cancer admitted solely for minor procedures, chemotherapy, stem-cell transplantation, or bone marrow transplantation.
Preventing VTE Recurrence
When a patient with cancer is diagnosed with VTE, rivaroxaban or LMWH should be considered for initial treatment, with unfractionated heparin and fondaparinux (Arixtra) as acceptable alternatives. Anticoagulation with LMWH, edoxaban, or rivaroxaban is recommended for at least six months after initial VTE. Vitamin K antagonists are less effective than LMWH or DOAC treatment at preventing recurrence. Extending treatment for an additional six to 12 months in high-risk patients with active cancer is recommended based on studies showing a 4% recurrence risk from six to 12 months after VTE, similar to the major bleeding risk from anticoagulation during this period.
Incidentally identified VTE in patients with cancer should be treated the same way as symptomatic VTE because similar outcomes occur. This recommendation does not apply to subsegmental pulmonary embolism, splanchnic thrombi, or visceral vein thrombi because evidence is insufficient.
VTE Recurrence Despite Anticoagulation
If VTE occurs despite anticoagulation, treatment adherence, adequacy of the dose, heparin-induced thrombocytopenia, and possible mechanical compression caused by the malignancy should be assessed. Other options include higher doses of LMWH or switching to a DOAC, although evidence to support these changes is absent.
Recurrent VTE is a common indication for vena cava filter placement, yet observational studies show long-term harm and no survival advantage. Filter placement has been shown to increase short- and long-term mortality and is given a weak recommendation by the group.
Editor's Note: The numbers needed to treat and to harm reported in this summary were calculated by the author based on raw data provided in the original guideline.
The views expressed are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.
Guideline source: American Society of Clinical Oncology
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: J Clin Oncol. February 10, 2020;38(5):496–520
