Am Fam Physician. 2020;102(6):363-366
Related Putting Prevention into Practice: Screening for Hepatitis C Virus Infection in Adolescents and Adults
As published by the USPSTF.
Summary of Recommendation and Evidence
The USPSTF recommends screening for hepatitis C virus (HCV) infection in adults aged 18 to 79 years (Table 1). B recommendation.
See Table 1 for a more detailed summary of the recommendation for clinicians.
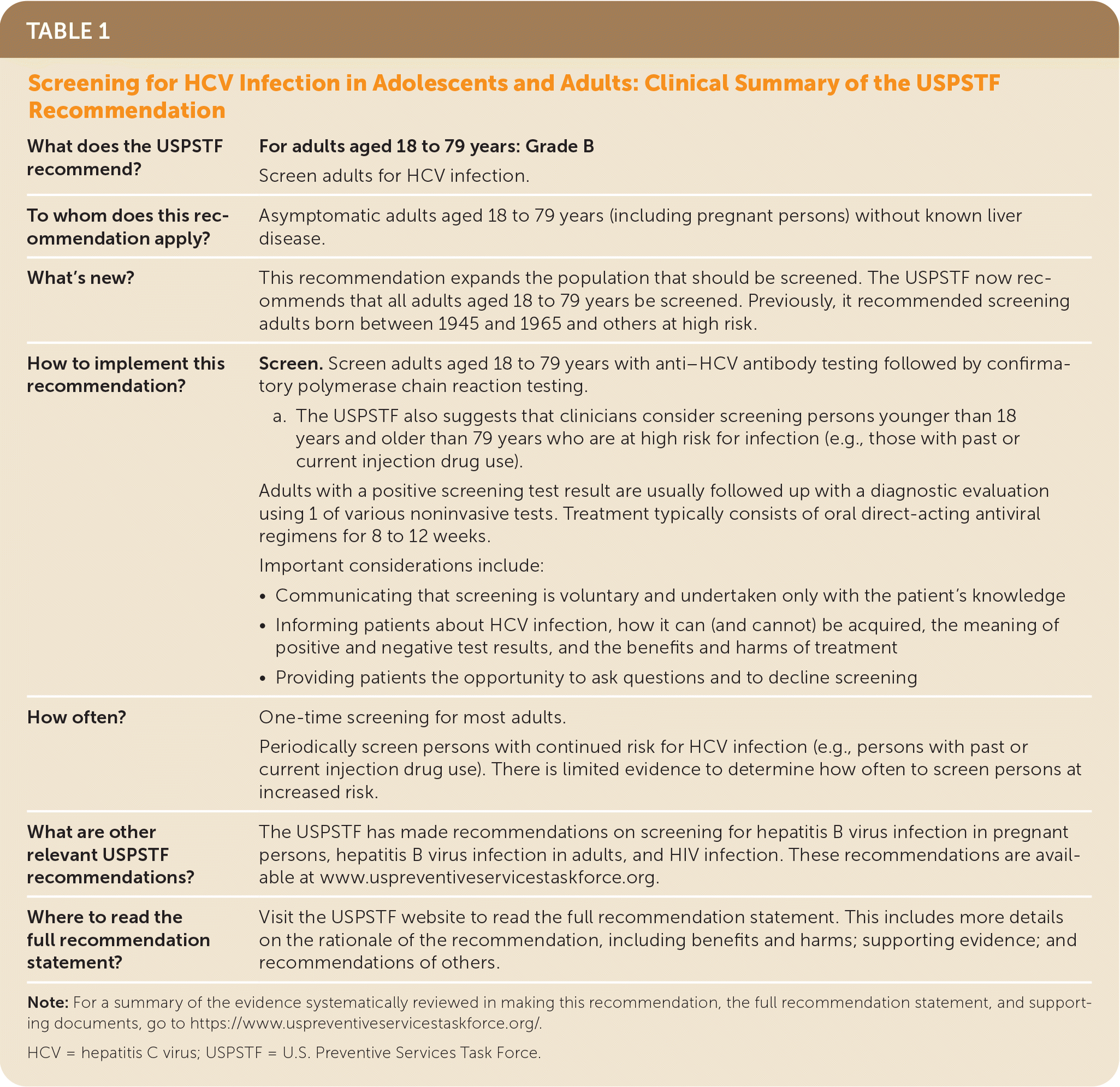
| What does the USPSTF recommend? | For adults aged 18 to 79 years: Grade B Screen adults for HCV infection. |
| To whom does this recommendation apply? | Asymptomatic adults aged 18 to 79 years (including pregnant persons) without known liver disease. |
| What's new? | This recommendation expands the population that should be screened. The USPSTF now recommends that all adults aged 18 to 79 years be screened. Previously, it recommended screening adults born between 1945 and 1965 and others at high risk. |
| How to implement this recommendation? | Screen. Screen adults aged 18 to 79 years with anti–HCV antibody testing followed by confirmatory polymerase chain reaction testing.
Adults with a positive screening test result are usually followed up with a diagnostic evaluation using 1 of various noninvasive tests. Treatment typically consists of oral direct-acting antiviral regimens for 8 to 12 weeks. Important considerations include:
|
| How often? | One-time screening for most adults. Periodically screen persons with continued risk for HCV infection (e.g., persons with past or current injection drug use). There is limited evidence to determine how often to screen persons at increased risk. |
| What are other relevant USPSTF recommendations? | The USPSTF has made recommendations on screening for hepatitis B virus infection in pregnant persons, hepatitis B virus infection in adults, and HIV infection. These recommendations are available at www.uspreventiveservicestaskforce.org. |
| Where to read the full recommendation statement? | Visit the USPSTF website to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. |
Importance
HCV is the most common chronic blood-borne pathogen in the United States and is a leading cause of complications from chronic liver disease.1 HCV infection is associated with more deaths than the top 60 other reportable infectious diseases combined, including HIV.2 The most important risk factor for HCV infection is past or current injection drug use.1 In the United States, an estimated 4.1 million persons have past or current HCV infection (i.e., they test positive for the anti-HCV antibody). Of these persons who test positive for the anti-HCV antibody, approximately 2.4 million have current infections based on testing with molecular assays for HCV RNA.1,3–5 The estimated prevalence of chronic HCV infection is approximately 1.0% (2013 to 2016).6 An estimated 44,700 new HCV infections occurred in the United States in 2017.7 Cases of acute HCV infection have increased approximately 3.8-fold (2010 to 2017) over the last decade because of increasing injection drug use and improved surveillance.7 The most rapid increase in acute HCV incidence has been in young adults aged 20 to 39 years who inject drugs, with increases in both sexes but more pronounced in men.7 Rates increased especially in American Indian/Alaska Native and non-Hispanic white populations.7
Assessment of Magnitude of Net Benefit
The USPSTF concludes with moderate certainty that screening for HCV infection in adults aged 18 to 79 years has substantial net benefit.
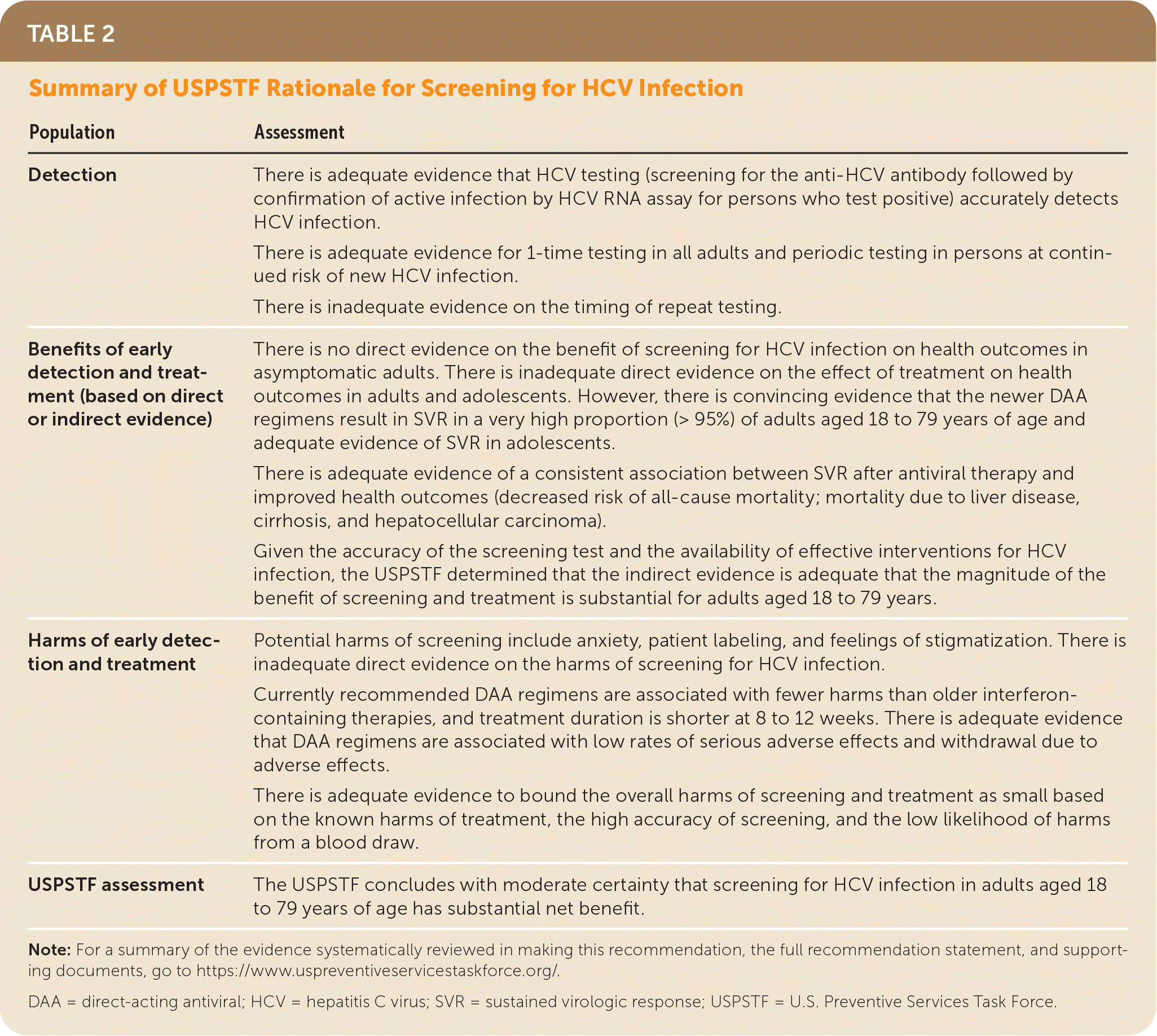
| Population | Assessment |
|---|---|
| Detection | There is adequate evidence that HCV testing (screening for the anti-HCV antibody followed by confirmation of active infection by HCV RNA assay for persons who test positive) accurately detects HCV infection. There is adequate evidence for 1-time testing in all adults and periodic testing in persons at continued risk of new HCV infection. There is inadequate evidence on the timing of repeat testing. |
| Benefits of early detection and treatment (based on direct or indirect evidence) | There is no direct evidence on the benefit of screening for HCV infection on health outcomes in asymptomatic adults. There is inadequate direct evidence on the effect of treatment on health outcomes in adults and adolescents. However, there is convincing evidence that the newer DAA regimens result in SVR in a very high proportion (> 95%) of adults aged 18 to 79 years of age and adequate evidence of SVR in adolescents. There is adequate evidence of a consistent association between SVR after antiviral therapy and improved health outcomes (decreased risk of all-cause mortality; mortality due to liver disease, cirrhosis, and hepatocellular carcinoma). Given the accuracy of the screening test and the availability of effective interventions for HCV infection, the USPSTF determined that the indirect evidence is adequate that the magnitude of the benefit of screening and treatment is substantial for adults aged 18 to 79 years. |
| Harms of early detection and treatment | Potential harms of screening include anxiety, patient labeling, and feelings of stigmatization. There is inadequate direct evidence on the harms of screening for HCV infection. Currently recommended DAA regimens are associated with fewer harms than older interferon-containing therapies, and treatment duration is shorter at 8 to 12 weeks. There is adequate evidence that DAA regimens are associated with low rates of serious adverse effects and withdrawal due to adverse effects. There is adequate evidence to bound the overall harms of screening and treatment as small based on the known harms of treatment, the high accuracy of screening, and the low likelihood of harms from a blood draw. |
| USPSTF assessment | The USPSTF concludes with moderate certainty that screening for HCV infection in adults aged 18 to 79 years of age has substantial net benefit. |
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to all asymptomatic adults aged 18 to 79 years without known liver disease.
ASSESSMENT OF RISK
Although all adults aged 18 to 79 years should be screened, a number of risk factors increase risk. The most important risk factor for HCV infection is past or current injection drug use. In the United States, recent increases in HCV incidence have predominantly been among young persons who inject drugs.1,9 Approximately one-third of persons who inject drugs aged 18 to 30 years are infected with HCV, and 70% to 90% of older persons who inject drugs are infected.9 Clinicians may want to consider screening in adolescents younger than 18 years and in adults older than 79 years who are at high risk (e.g., past or current injection drug use).
Pregnant adults should be screened. HCV prevalence has doubled in women aged 15 to 44 years from 2006 to 2014.1,10,11 From 2011 to 2014, 0.73% of pregnant women tested had an HCV infection, with a 68% increase in the proportion of infants born to HCV-infected mothers.1,10 Approximately 1,700 infected infants are born annually to 29,000 HCV-infected mothers.1,11 Because of the increasing prevalence of HCV in women aged 15 to 44 years and in infants born to HCV-infected mothers, clinicians may want to consider screening pregnant persons younger than 18 years.
SCREENING TESTS
Screening with anti-HCV antibody testing followed by polymerase chain reaction testing for HCV RNA is accurate for identifying patients with chronic HCV infection.9 Currently, diagnostic evaluations are often performed with various noninvasive tests that have lower risk for harm than liver biopsy for diagnosing fibrosis stage or cirrhosis in persons who screen positive.12
Among patients with abnormal results on liver function tests (measurement of aspartate aminotransferase, alanine aminotransferase, or bilirubin levels) who were tested for reasons other than HCV screening, finding the cause of the abnormality often includes testing for HCV infection and is considered case finding rather than screening; therefore, it is outside the scope of this recommendation.
SCREENING INTERVALS
Most adults need to be screened only once. Persons with continued risk for HCV infection (e.g., persons who inject drugs) should be screened periodically. There is limited information about the specific screening interval that should occur in persons who continue to be at risk for new HCV infection or how pregnancy changes the need for additional screening.
SCREENING IMPLEMENTATION
Important considerations for implementation of screening include (1) communicating to patients that screening is voluntary and undertaken only with the patient's knowledge and understanding that HCV screening is planned; (2) informing patients about HCV infection, how it can (and cannot) be acquired, the meaning of positive and negative test results, and the benefits and harms of treatment; and (3) providing patients the opportunity to ask questions and to decline screening.
Some health care systems serving insured populations, some academic medical centers, and the Veterans Health Administration have achieved high rates of HCV screening and treatment. However, national HCV screening rates in community health centers and from the National Health Interview Study were 8.3% and 17.3%, respectively; 1 study of 4 safety-net primary care practices serving low-income and uninsured or underserved populations found that only 0.8% of persons born in 1945 through 1965 were screened over a 1-year period.13 Implementation of successful screening may require addressing various barriers to screening and treatment in diverse populations, such as the uninsured.
TREATMENT
The purpose of antiviral treatment regimens for HCV infection is to prevent long-term health complications of chronic HCV infection (e.g., cirrhosis, liver failure, hepatocellular carcinoma).
Currently, all oral direct-acting antiviral regimens without interferon have been accepted as the standard treatment for chronic HCV infection. Antiviral therapy is not generally considered during pregnancy because of the lack of data on the safety of newer direct-acting antiviral regimens during pregnancy and breastfeeding.14,15
ADDITIONAL TOOLS AND RESOURCES
The Centers for Disease Control and Prevention provides strategies for implementing a testing program and additional risk factors at https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm.16
OTHER RELATED USPSTF RECOMMENDATIONS
This recommendation statement was first published in JAMA. 2020;323(10):970–975.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening#fullrecommendationstart.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.