Am Fam Physician. 2020;102(6):373-374
Author disclosure: No relevant financial affiliations.
Lefamulin (Xenleta) is an antibiotic recently developed for treating community-acquired bacterial pneumonia in adults. It is a pleuromutilin, a class of antibiotic that inhibits bacterial protein synthesis. Although not recommended by the Infectious Diseases Society of America guidelines for community-acquired pneumonia, it is mentioned as an option that requires further research.1,2
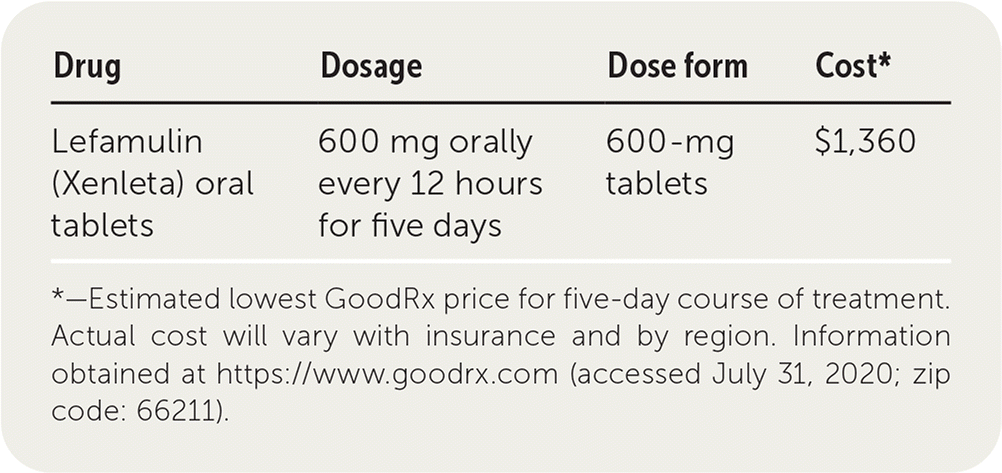
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lefamulin (Xenleta) oral tablets | 600 mg orally every 12 hours for five days | 600-mg tablets | $1,360 |
Safety
Similar to azithromycin (Zithromax), clarithromycin (Biaxin), and fluoroquinolones, lefamulin has the potential to prolong the QT interval and should not be used in patients taking medications that also affect the QT interval or cardiac conduction. Lefamulin has been shown to cause risk of fetal harm in animal studies, and it should not be used in pregnant patients.
Lefamulin has the potential to interact with multiple medications metabolized in the liver with the CYP3A4 enzyme, including phenytoin (Dilantin), rifampin, and azole antifungals. This may decrease the effectiveness of lefamulin and increase the risk of adverse effects.3
Tolerability
In clinical studies, diarrhea, nausea, and vomiting were more common with oral lefamulin than with oral moxifloxacin (Avelox). Diarrhea occurred in 12% of patients treated with oral lefamulin compared with 1% of patients receiving oral moxifloxacin. Administration site reactions with intravenous lefamulin occurred in 7.7% of patients vs. 3.7% of patients treated with intravenous moxifloxacin.
Effectiveness
In laboratory testing, lefamulin is effective against typical and atypical pathogens in patients with community-acquired bacterial pneumonia, including Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus, Haemophilus influenzae, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.
Lefamulin has been compared with moxifloxacin in two randomized, double-blind trials of 1,289 patients with clinical and radiographic evidence of community-acquired bacterial pneumonia.4,5 Hospitalized patients were started on intravenous lefamulin therapy every 12 hours with the option of switching to oral therapy after at least three days in the Lefamulin Evaluation Against Pneumonia (LEAP 1) trial.4 In the LEAP 2 trial, outpatients were randomized to receive oral lefamulin every 12 hours for five days compared with oral moxifloxacin daily for seven days.5 Primary effectiveness was demonstrated by improvement in at least two symptoms (cough, sputum production, chest pain, dyspnea) within four days. The secondary outcome included was investigator assessment of clinical response five to 10 days after the last dose. Both of these end points demonstrated that lefamulin was non-inferior to moxifloxacin, with no statistical differences in effectiveness between the treatment groups (87.3% vs. 90.2%, respectively, in the intravenous groups, and 90.8% in both oral groups). In patients with suspected methicillin-resistant S. aureus, lefamulin was as effective as moxifloxacin in combination with linezolid (Zyvox); however, all staphylococcus isolates in the study were methicillin-sensitive.
Although lefamulin was determined to be non-inferior to moxifloxacin, it is unknown whether this medication offers any clinical benefits in patients in whom other antibiotics have previously failed. It is also unclear if it is effective for health-care–acquired pneumonia or has benefits over existing therapies in terms of shortening length of hospital stay or decreasing mortality.
Price
The cost of a five-day course of oral lefamulin (10 tablets) is $1,360. This is substantially more expensive than other first-line drugs used to treat community-acquired bacterial pneumonia. Lefamulin tablets are available only at designated pharmacies. Switching to the oral form of lefamulin at discharge may require prior authorization to secure insurance coverage for continuation of oral therapy.
Simplicity
Oral lefamulin should be taken whole with a full glass of water. Patients should be instructed to take it at least one hour before a meal or two hours after a meal, because food can decrease bioavailability.
Bottom Line
Lefamulin may be considered for patients hospitalized with community-acquired bacterial pneumonia. However, it is considerably more expensive than other antibiotics and may require additional authorization to obtain. Lefamulin should not be used as a first-line treatment for community-acquired bacterial pneumonia, but it may be an option for patients in whom resistance is a concern or who are unable to take currently recommended antibiotics.