
Am Fam Physician. 2020;102(8):505-507
Key Points for Practice
• Influenza vaccination continues to be recommended for all people six months and older and is especially important during the COVID-19 pandemic to reduce health care system burden.
• Vaccination in September or October is most effective, especially for older adults.
• Two new high-dose quadrivalent vaccines are available for patients 65 years and older.
From the AFP Editors
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) has released its recommendations for routine influenza vaccination in the 2020–2021 season. Updates this year include the antigenic composition of seasonal influenza vaccines available in the United States, the addition of two new influenza vaccines for use in people 65 years and older, and new contraindications and precautions for the use of live attenuated influenza vaccine (LAIV).
Because the 2020–2021 influenza season will coincide with the coronavirus disease 2019 (COVID-19) pandemic, vaccination of people six months and older is of particular importance to reduce symptoms that could be confused with those of COVID-19 and to alleviate stress on the health care system. During the 2017–2018 influenza season, which had an unusually long duration of widespread influenza activity and high hospitalization rates, vaccination was calculated to have prevented an estimated 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 deaths.
Influenza vaccination is recommended for all people six months and older who do not have contraindications. Vaccination is most effective if received by the end of October, although a vaccine administered in December or later is likely still beneficial. Children six months to eight years of age who are not known to have received at least two doses of influenza vaccine at least four weeks apart before July 1, 2020, require two doses of vaccine this season and should receive the first dose when the vaccine is available so the second dose can be administered by the end of October. For people who require only one dose this season, immunity will be best with vaccination in September or October, particularly among older adults. Influenza vaccination can be delayed in people with suspected or confirmed COVID-19 until they are no longer acutely ill.
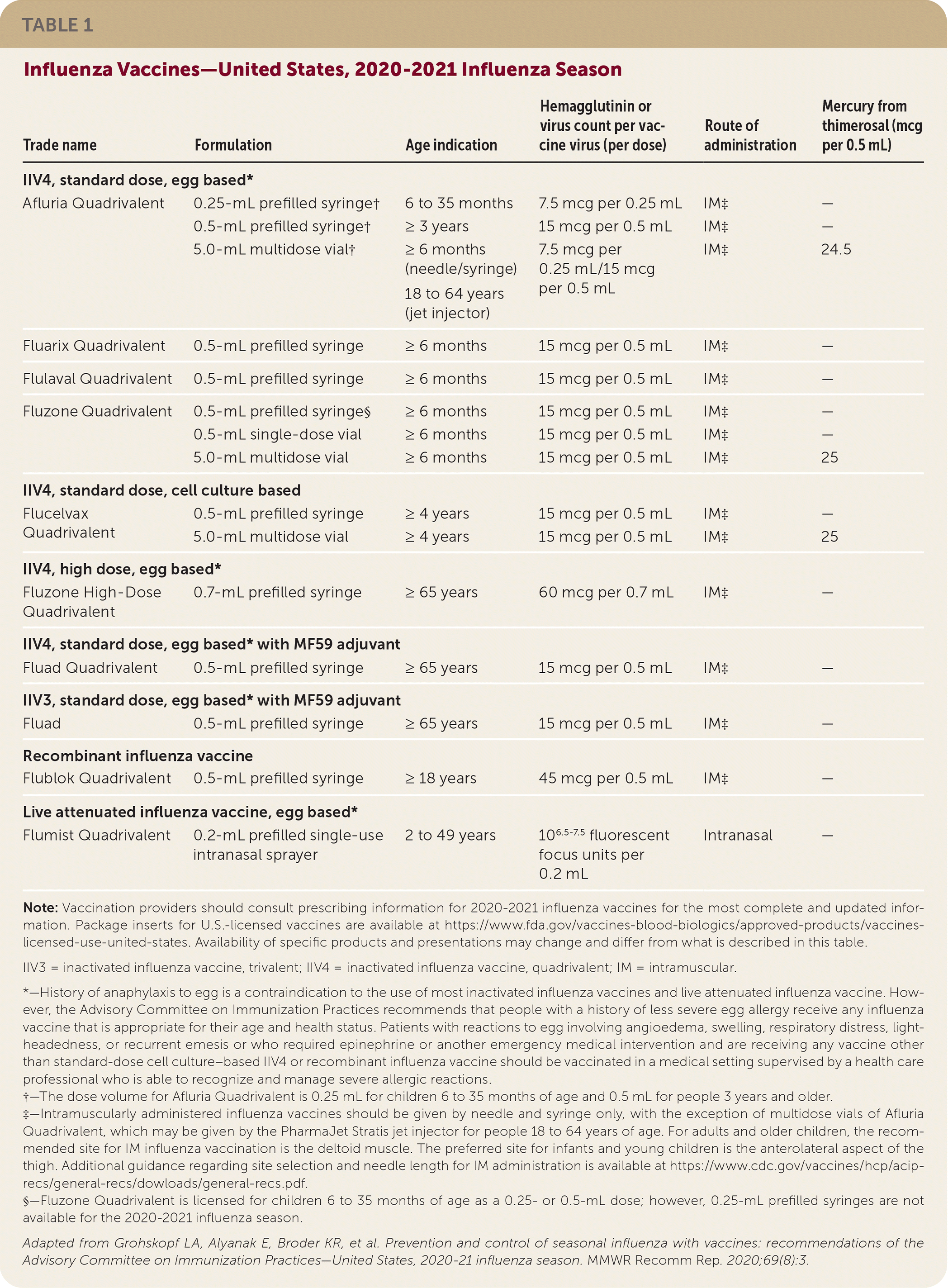
Inactivated influenza vaccines, recombinant influenza vaccine, and LAIV are available for the 2020–2021 influenza season (Table 1). Standard-dose and high-dose, unadjuvanted, inactivated influenza vaccines are available in quadrivalent formulations. Adjuvanted inactivated vaccines are available in trivalent and quadrivalent formulations. Recombinant influenza vaccine and LAIV are available in quadrivalent formulations. For the 2020–2021 influenza season, all inactivated vaccines are egg based, except for Flucelvax Quadrivalent, which is cell culture based, and Flublock Quadrivalent, a recombinant vaccine. People with egg allergy can receive any vaccine, although those with a history of severe allergy to egg should be supervised in a health care setting after administration.
| Trade name | Formulation | Age indication | Hemagglutinin or virus count per vaccine virus (per dose) | Route of administration | Mercury from thimerosal (mcg per 0.5 mL) |
|---|---|---|---|---|---|
| IIV4, standard dose, egg based* | |||||
| Afluria Quadrivalent | 0.25-mL prefilled syringe† | 6 to 35 months | 7.5 mcg per 0.25 mL | IM‡ | — |
| 0.5-mL prefilled syringe† | ≥ 3 years | 15 mcg per 0.5 mL | IM‡ | — | |
| 5.0-mL multidose vial† | ≥ 6 months (needle/syringe) | 7.5 mcg per 0.25 mL/15 mcg per 0.5 mL | IM‡ | 24.5 | |
| 18 to 64 years (jet injector) | |||||
| Fluarix Quadrivalent | 0.5-mL prefilled syringe | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — |
| Flulaval Quadrivalent | 0.5-mL prefilled syringe | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — |
| Fluzone Quadrivalent | 0.5-mL prefilled syringe§ | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — |
| 0.5-mL single-dose vial | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — | |
| 5.0-mL multidose vial | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | 25 | |
| IIV4, standard dose, cell culture based | |||||
| Flucelvax | 0.5-mL prefilled syringe | ≥ 4 years | 15 mcg per 0.5 mL | IM‡ | — |
| Quadrivalent | 5.0-mL multidose vial | ≥ 4 years | 15 mcg per 0.5 mL | IM‡ | 25 |
| IIV4, high dose, egg based* | |||||
| Fluzone High-Dose Quadrivalent | 0.7-mL prefilled syringe | ≥ 65 years | 60 mcg per 0.7 mL | IM‡ | — |
| IIV4, standard dose, egg based* with MF59 adjuvant | |||||
| Fluad Quadrivalent | 0.5-mL prefilled syringe | ≥ 65 years | 15 mcg per 0.5 mL | IM‡ | — |
| IIV3, standard dose, egg based* with MF59 adjuvant | |||||
| Fluad | 0.5-mL prefilled syringe | ≥ 65 years | 15 mcg per 0.5 mL | IM‡ | — |
| Recombinant influenza vaccine | |||||
| Flublok Quadrivalent | 0.5-mL prefilled syringe | ≥ 18 years | 45 mcg per 0.5 mL | IM‡ | — |
| Live attenuated influenza vaccine, egg based* | |||||
| Flumist Quadrivalent | 0.2-mL prefilled single-use intranasal sprayer | 2 to 49 years | 106.5–7.5 fluorescent focus units per 0.2 mL | Intranasal | — |
The composition of the 2020–2021 U.S. influenza vaccines includes updates to the influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B/Victoria lineage components. These updated components are included in the trivalent and quadrivalent vaccines. Quadrivalent vaccines include an additional influenza B virus component from the B/Yamagata lineage, which is unchanged from the 2019–2020 season.
Two new vaccines are available this season for older adults: Fluzone High-Dose Quadrivalent, which replaces the trivalent high-dose formulation, and Fluad Quadrivalent.
LAIV may be used intranasally in children at least two years of age and in adults up to 49 years of age. LAIV should not be used in pregnant women. Newly added contraindications for the use of LAIV include anatomic and functional asplenia; active communication between the cerebrospinal fluid and oropharynx, nasopharynx, nose, ear, or other cranial cerebrospinal fluid leak; and cochlear implants. For a full list of vaccine contraindications, see Table 2 at https://www.cdc.gov/mmwr/volumes/69/rr/rr6908a1.htm.
People of all ages are susceptible to seasonal influenza, but vaccination is particularly important for those at increased risk of severe complications or influenza-related hospitalizations, and those who live with or care for such people. When vaccine supply is limited, vaccination efforts should target the following groups:
Higher-risk people:
○ Adults 50 years and older
○ American Indians/Alaska Natives
○ Children six to 59 months of age
○ Children up to 18 years of age who are receiving aspirin- or salicylate-containing medications (risk of Reye syndrome after influenza virus infection)
○ Immunocompromised people
○ People with a body mass index of 40 kg per m2 or greater
○ People with chronic pulmonary, cardiovascular, renal, hepatic, neurologic, hematologic, or metabolic disorders other than uncomplicated hypertension
○ Pregnant women
○ Residents of long-term care facilities
Household contacts and caregivers of higher-risk people
Health care professionals, including students and staff who could be exposed to infectious agents
Guideline source: Advisory Committee on Immunization Practices
Evidence rating system used? No
Systematic literature search described? No
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: MMWR Recomm Rep. August 21, 2020;69(8): 1–24
Available at: https://www.cdc.gov/mmwr/volumes/69/rr/rr6908a1.htm
