
Am Fam Physician. 2021;103(3):183-185
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
In adults with suspected cognitive impairment, what is the utility of brief cognitive testing in detecting clinical Alzheimer-type dementia (ATD) and distinguishing it from mild cognitive impairment (MCI) or normal cognition?
Evidence-Based Answer
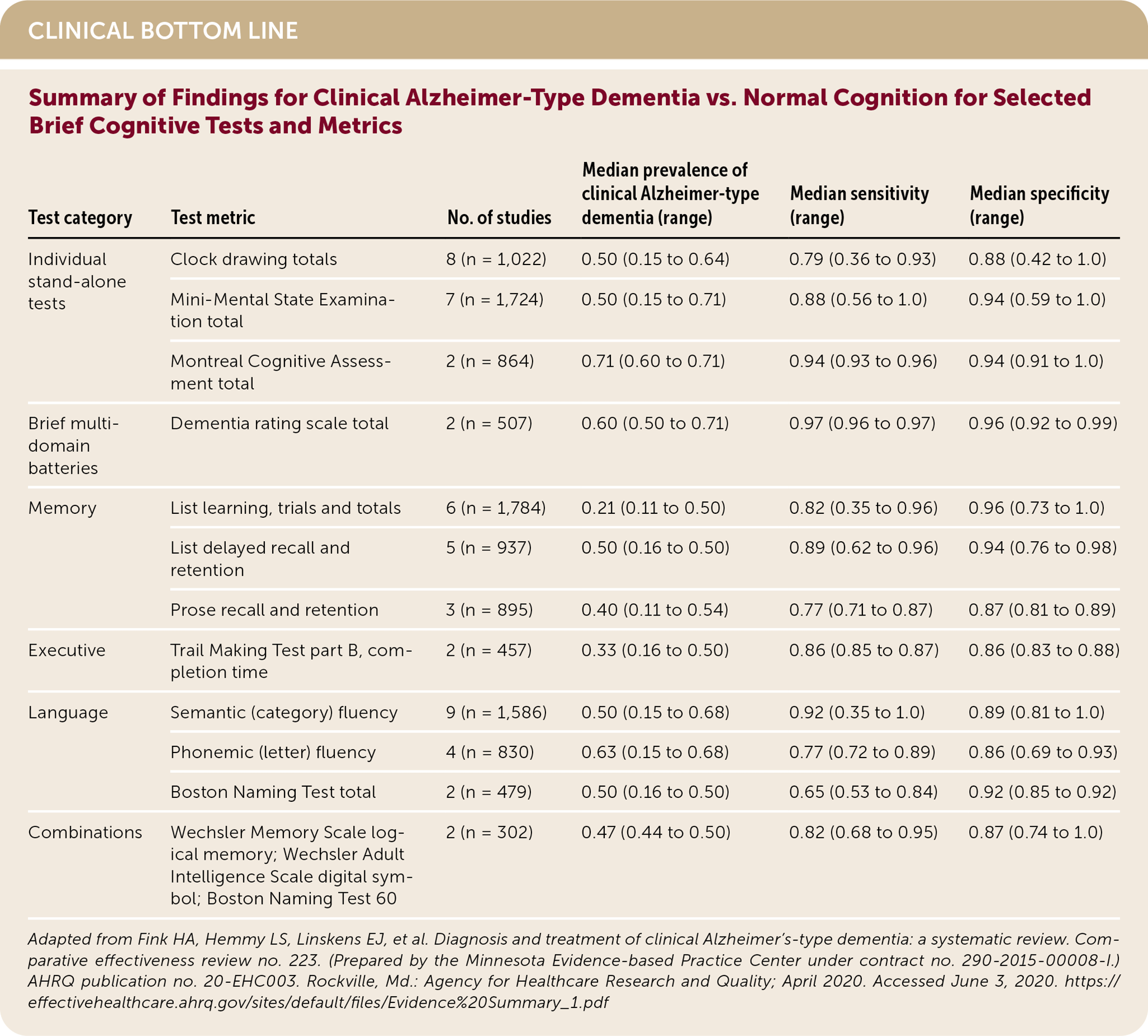
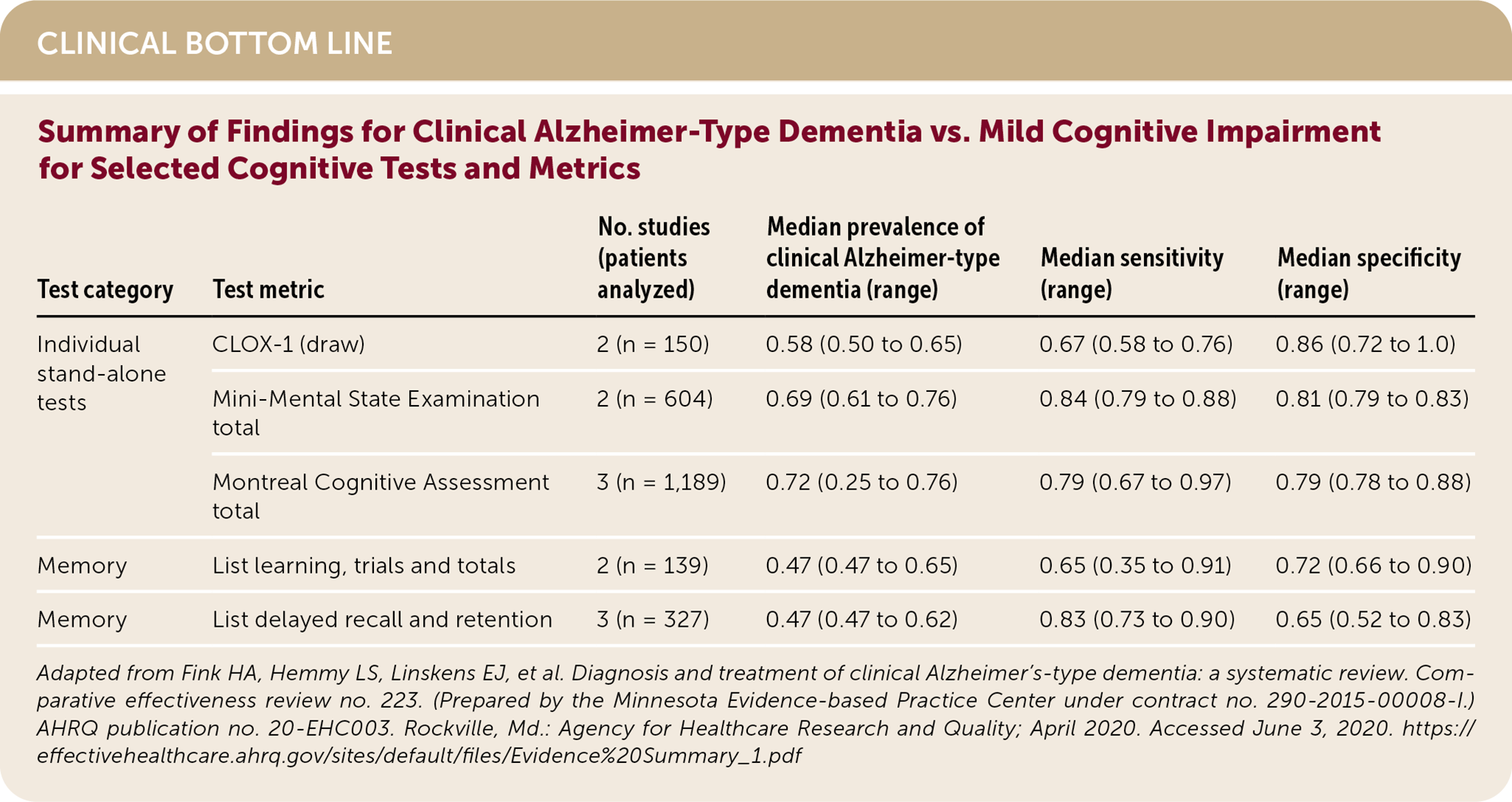
The Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), list learning memory test, list delayed recall memory test, and the semantic fluency language test have sensitivities and specificities that are 0.80 or greater in distinguishing clinical ATD from normal cognition. The MoCA is the only stand-alone test with sensitivity and specificity greater than 0.90 for this comparison. (Strength of Recommendation [SOR]: C, based on disease-oriented evidence.) Brief cognitive tests are less accurate in distinguishing clinical ATD from MCI compared with distinguishing it from normal cognitive function. (SOR: C, based on disease-oriented evidence.) Brief cognitive testing alone is insufficient to definitively detect or diagnose clinical ATD.1 (SOR: C, based on disease-oriented evidence.)
| Test category | Test metric | No. of studies | Median prevalence of clinical Alzheimer-type dementia (range) | Median sensitivity (range) | Median specificity (range) |
|---|---|---|---|---|---|
| Individual stand-alone tests | Clock drawing totals | 8 (n = 1,022) | 0.50 (0.15 to 0.64) | 0.79 (0.36 to 0.93) | 0.88 (0.42 to 1.0) |
| Mini-Mental State Examination total | 7 (n = 1,724) | 0.50 (0.15 to 0.71) | 0.88 (0.56 to 1.0) | 0.94 (0.59 to 1.0) | |
| Montreal Cognitive Assessment total | 2 (n = 864) | 0.71 (0.60 to 0.71) | 0.94 (0.93 to 0.96) | 0.94 (0.91 to 1.0) | |
| Brief multidomain batteries | Dementia rating scale total | 2 (n = 507) | 0.60 (0.50 to 0.71) | 0.97 (0.96 to 0.97) | 0.96 (0.92 to 0.99) |
| Memory | List learning, trials and totals | 6 (n = 1,784) | 0.21 (0.11 to 0.50) | 0.82 (0.35 to 0.96) | 0.96 (0.73 to 1.0) |
| List delayed recall and retention | 5 (n = 937) | 0.50 (0.16 to 0.50) | 0.89 (0.62 to 0.96) | 0.94 (0.76 to 0.98) | |
| Prose recall and retention | 3 (n = 895) | 0.40 (0.11 to 0.54) | 0.77 (0.71 to 0.87) | 0.87 (0.81 to 0.89) | |
| Executive | Trail Making Test part B, completion time | 2 (n = 457) | 0.33 (0.16 to 0.50) | 0.86 (0.85 to 0.87) | 0.86 (0.83 to 0.88) |
| Language | Semantic (category) fluency | 9 (n = 1,586) | 0.50 (0.15 to 0.68) | 0.92 (0.35 to 1.0) | 0.89 (0.81 to 1.0) |
| Phonemic (letter) fluency | 4 (n = 830) | 0.63 (0.15 to 0.68) | 0.77 (0.72 to 0.89) | 0.86 (0.69 to 0.93) | |
| Boston Naming Test total | 2 (n = 479) | 0.50 (0.16 to 0.50) | 0.65 (0.53 to 0.84) | 0.92 (0.85 to 0.92) | |
| Combinations | Wechsler Memory Scale logical memory; Wechsler Adult Intelligence Scale digital symbol; Boston Naming Test 60 | 2 (n = 302) | 0.47 (0.44 to 0.50) | 0.82 (0.68 to 0.95) | 0.87 (0.74 to 1.0) |
| Test category | Test metric | No. of studies | Median prevalence of clinical Alzheimer-type dementia (range) | Median sensitivity (range) | Median specificity (range) |
|---|---|---|---|---|---|
| Individual stand-alone tests | CLOX-1 (draw) | 2 (n = 150) | 0.58 (0.50 to 0.65) | 0.67 (0.58 to 0.76) | 0.86 (0.72 to 1.0) |
| Mini-Mental State Examination total | 2 (n = 604) | 0.69 (0.61 to 0.76) | 0.84 (0.79 to 0.88) | 0.81 (0.79 to 0.83) | |
| Montreal Cognitive Assessment total | 3 (n = 1,189) | 0.72 (0.25 to 0.76) | 0.79 (0.67 to 0.97) | 0.79 (0.78 to 0.88) | |
| Memory | List learning, trials and totals | 2 (n = 139) | 0.47 (0.47 to 0.65) | 0.65 (0.35 to 0.91) | 0.72 (0.66 to 0.90) |
| Memory | List delayed recall and retention | 3 (n = 327) | 0.47 (0.47 to 0.62) | 0.83 (0.73 to 0.90) | 0.65 (0.52 to 0.83) |
Practice Pointers
In the United States, 5.8 million people 65 years and older (11.4% of this age group) are estimated to have clinical ATD.2,3 Deaths from stroke and heart disease have decreased over the past two decades while the number of deaths associated with clinical ATD has increased, making it the sixth leading cause of death. Direct costs related to the care of clinical ATD total $305 billion annually, and caregiving by unpaid family members is valued at $244 billion.2 Given the prevalence, burden, and associated costs of clinical ATD, tools that assist in diagnosing the disease may inform care and improve outcomes.
This Agency for Healthcare Research and Quality review included 57 English-language studies that used validated cognitive tests for distinguishing clinical ATD from normal cognition. Only studies that used established criteria for diagnosing clinical ATD and that were judged to have a low or medium risk of bias were included. More than 80% of patients in these studies were White, and 60% were women. Most studies were retrospective and used the study cohort to set the level of discrimination between clinical ATD and MCI or normal cognition, as opposed to using prespecified cutoff values.1,4
The authors found that most stand-alone cognitive tests had high sensitivity and specificity for clinical ATD vs. normal cognitive function. The MoCA (two studies, 864 participants) had a sensitivity of 0.94 (range = 0.93 to 0.96) and specificity of 0.94 (range = 0.91 to 1.0) in a cohort with a median clinical ATD prevalence of 71%. The MMSE (seven studies, 1,724 participants) had a sensitivity of 0.88 (range = 0.56 to 1.0) and specificity of 0.94 (range = 0.59 to 1.0) in a cohort with a median clinical ATD prevalence of 50%. Clock drawing tests (eight studies, 1,022 participants) had a sensitivity of 0.79 (range = 0.36 to 0.93) and specificity of 0.88 (range = 0.42 to 1.0) in a cohort with a median clinical ATD prevalence of 50%.1 The MoCA and MMSE are proprietary, but many clock drawing tests are free and can be used as a stand-alone test or as part of other screening tools, such as the Mini-Cog (also free).
Among memory tests, list learning, list delayed recall, and prose recall and retention tests had sensitivities ranging from 0.77 to 0.89 and specificities from 0.87 to 0.96 in cohorts of patients with median clinical ATD prevalence of 21% to 50%. Other tests, such as the Trail Making Test and verbal fluency tests, had similar performance.1
The American Academy of Neurology (AAN) recommends assessing for cognitive impairment in individuals concerned about their memory or if their close contacts voice concern. The AAN stresses the use of validated screening tools followed by more comprehensive tools, such as neuropsychological testing, in those who screen positive.5 Although the U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to support routine screening for dementia or MCI in asymptomatic individuals, it determined that screening instruments are able to detect cognitive impairment in older adults. The USPSTF found that these brief cognitive tests are more accurate in distinguishing dementia from normal cognition than distinguishing MCI from normal cognition. The USPSTF found insufficient evidence that screening and early detection improve clinical decision-making or patient or caregiver outcomes.6
The AAN recommends that physicians caring for individuals with cognitive impairment review medication lists for medications known to cause impairment and discontinue them if possible. Physicians should also prescribe regular exercise, consider cognitive training, and discuss prognosis and long-term planning with patients.5
Editor's Note: Dr. Saguil is a contributing editor for AFP. The Mini-Mental State Examination (MMSE) had been freely available and widely disseminated after first being released in 1975. Starting in 2000, its authors (Dr. Folstein and others) began enforcing its copyright and in 2001 arranged for Psychological Assessment Resources (PAR) to manage worldwide rights. PAR insists that all users register with their site, complete a four-page permissions request form, and purchase MMSE forms ($99 for 50 forms) and a test manual ($114) [costs as of January 2021]. See https://www.parinc.com/Resources/Permissions-and-licensing. The creators of the Montreal Cognitive Assessment (MoCA) have followed suit by requiring training and certification to administer the test with restricted use starting in 2021. The training is one hour, and the cost is $125 for the initial two years of certification, which will then require renewal. See https://www.mocatest.org/. This commercialization of a cognitive screening test seems antithetical to the advancement of science and the practice of medicine. As long as copyright holders of these tools restrict their use, clinicians should know that there are alternatives to the MMSE and MoCA, including the Saint Louis University Mental Status examination. See https://www.slu.edu/medicine/internal-medicine/geriatric-medicine/aging-successfully/assessment-tools/mental-status-exam.php.—Jay Siwek, MD, Editor Emeritus; Sumi Sexton, MD, Editor-in-Chief
The views expressed in this article are the authors' and do not reflect official policy or position of the Uniformed Services University of the Health Sciences, Madigan Army Medical Center, the Department of Defense, or the U.S. government.
