
Am Fam Physician. 2021;103(6):337-343
Related letter to the Editor: Reevaluating the Measurement of Potency of Topical Corticosteroids
Author disclosure: No relevant financial affiliations.
Topical corticosteroids are an essential tool for treating inflammatory skin conditions such as psoriasis and atopic dermatitis. Topical corticosteroids are classified by strength and the risk of adverse effects such as atrophy, striae, rosacea, telangiectasias, purpura, and other cutaneous and systemic reactions. The risk of adverse effects increases with prolonged use, a large area of application, higher potency, occlusion, and application to areas of thinner skin such as the face and genitals. When prescribing topical corticosteroids for use in children, lower potencies and shorter durations should be used. Topical corticosteroids can work safely and effectively in patients who are pregnant or lactating. They are available in formulations such as ointments, creams, lotions, gels, foams, oils, solutions, and shampoos. The quantity of corticosteroid prescribed depends on the duration of treatment, the frequency of application, the skin location, and the total surface area treated. Correct patient application is critical to successful use. Patients may be taught application using the fingertip unit method. One fingertip unit is the amount of medication dispensed from the tip of the index finger to the crease of the distal interphalangeal joint and covers approximately 2% body surface area on an adult. Topical corticosteroids are applied once or twice per day for up to three weeks for super-high-potency corticosteroids or up to 12 weeks for high- or medium-potency corticosteroids. There is no specified time limit for low-potency topical corticosteroid use.
Effective treatment of dermatologic disorders depends on an accurate diagnosis based on history, physical examination, and appropriate diagnostic tests such as biopsy or skin scraping. Typical characteristics of skin diseases amenable to treatment with topical corticosteroids include inflammation, hyperproliferation, and immunologic etiology.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Use topical corticosteroids to treat psoriasis and atopic dermatitis.3,4 | A | Multiple randomized controlled trials and meta-analyses show consistent improvement with corticosteroid use |
| Use topical corticosteroids to treat inflammatory skin diseases, including lichen sclerosus, alopecia areata, vitiligo, bullous pemphigoid, phimosis, and aphthous ulcers.5–10 | B | Expert opinion, consensus guidelines, and inconsistent results from multiple small, well-controlled studies |
| Base the choice of a corticosteroid formulation on characteristics of the lesion, cost, and patient preference. Potential differences in strength between formulations should not be the main factor in choice.26–29 | C | Expert opinion and disease-oriented evidence |
| Teach patients how to apply topical corticosteroids using the fingertip unit method.33,34 | C | Expert opinion and consensus guidelines |
Topical corticosteroids are approved by the U.S. Food and Drug Administration for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.1 Table 1 lists skin conditions that respond to treatment with topical corticosteroids.2 This article discusses the use of topical corticosteroids on nonmucosal surfaces.
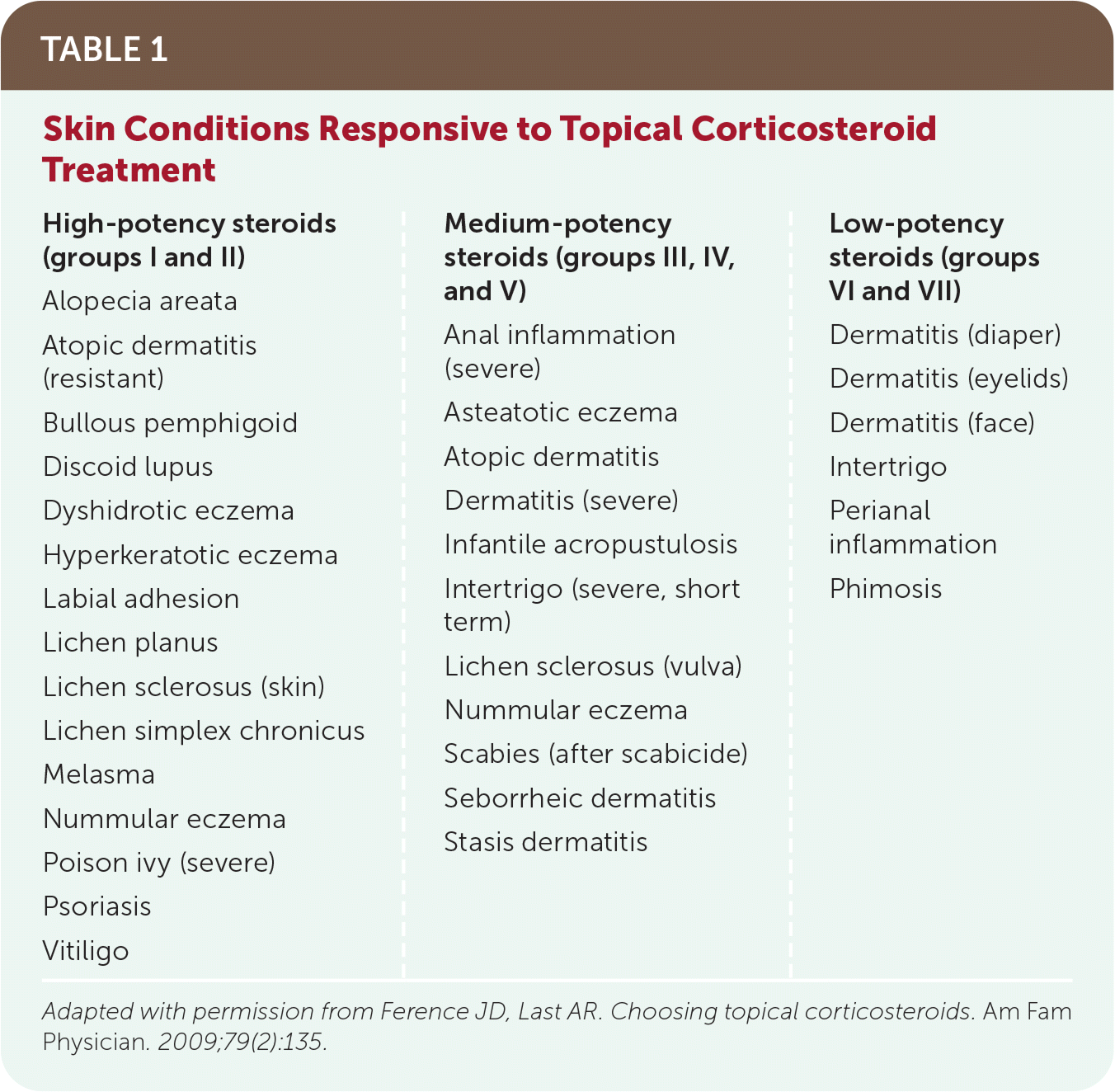
| High-potency steroids (groups I and II) Alopecia areata Atopic dermatitis (resistant) Bullous pemphigoid Discoid lupus Dyshidrotic eczema Hyperkeratotic eczema Labial adhesion Lichen planus Lichen sclerosus (skin) Lichen simplex chronicus Melasma Nummular eczema Poison ivy (severe) Psoriasis Vitiligo | Medium-potency steroids (groups III, IV, and V) Anal inflammation (severe) Asteatotic eczema Atopic dermatitis Dermatitis (severe) Infantile acropustulosis Intertrigo (severe, short term) Lichen sclerosus (vulva) Nummular eczema Scabies (after scabicide) Seborrheic dermatitis Stasis dermatitis | Low-potency steroids (groups VI and VII) Dermatitis (diaper) Dermatitis (eyelids) Dermatitis (face) Intertrigo Perianal inflammation Phimosis |
Despite widespread use of topical corticosteroids, high-quality research demonstrating their effectiveness exists for a relatively small number of diagnoses. Multiple systematic reviews support the use of medium- and high-potency topical corticosteroids for the treatment of atopic dermatitis, and intermittent use to prevent a recurrence.3 A Cochrane review found evidence to support the use of topical corticosteroids for the treatment of psoriasis, with super-high-potency corticosteroids being the most effective.4
A limited number of double-blind, randomized controlled trials also support topical corticosteroid use for the treatment of lichen sclerosus,5 alopecia areata,6 vitiligo,7 bullous pemphigoid,8 phimosis,9 and aphthous ulcers.10 Lower-level evidence supports topical corticosteroid use for other conditions, including melasma,11 infantile acropustulosis,12 and prepubertal labial adhesions.13
Potency
The strength of a topical corticosteroid is a factor of the agent and concentration. Certain compounds are inherently more potent than others, and potency increases as the concentration of corticosteroid increases.
Topical corticosteroids are grouped into seven classes based on their performance on the vasoconstriction assay, which tests the ability of the corticosteroid to blanch skin. Many can fall into multiple classes based on their concentration. Evidence from small, nonrandomized trials supports the correlation between strength that is measured by the vasoconstriction assay and clinical effectiveness or risk of harm.14 The effectiveness of the corticosteroid may differ depending on the thickness of application, the condition being treated, duration of treatment, and if the medication is a brand name vs. generic formulation.15,16
Although it is not practical to memorize an exhaustive list of corticosteroids, clinicians should be well-acquainted with at least one in each of the low-, medium-, high-, and super-high-potency classes (Table 21,17–19). This is sufficient to treat most corticosteroid-responsive skin conditions that present in primary care. Low-potency corticosteroids are useful in children or for mild, widespread disease, or on thin skin areas such as the face and groin. Medium- to high-potency corticosteroids are used for most areas on the body, such as the trunk and extremities. Super-high-potency corticosteroids are useful for severe disease and in areas of thick skin such as the palms, soles, or psoriatic lesions. Except in rare circumstances and for short durations, super-high-potency corticosteroids should not be used in children, under occlusion, or on the face, groin, or skinfolds.
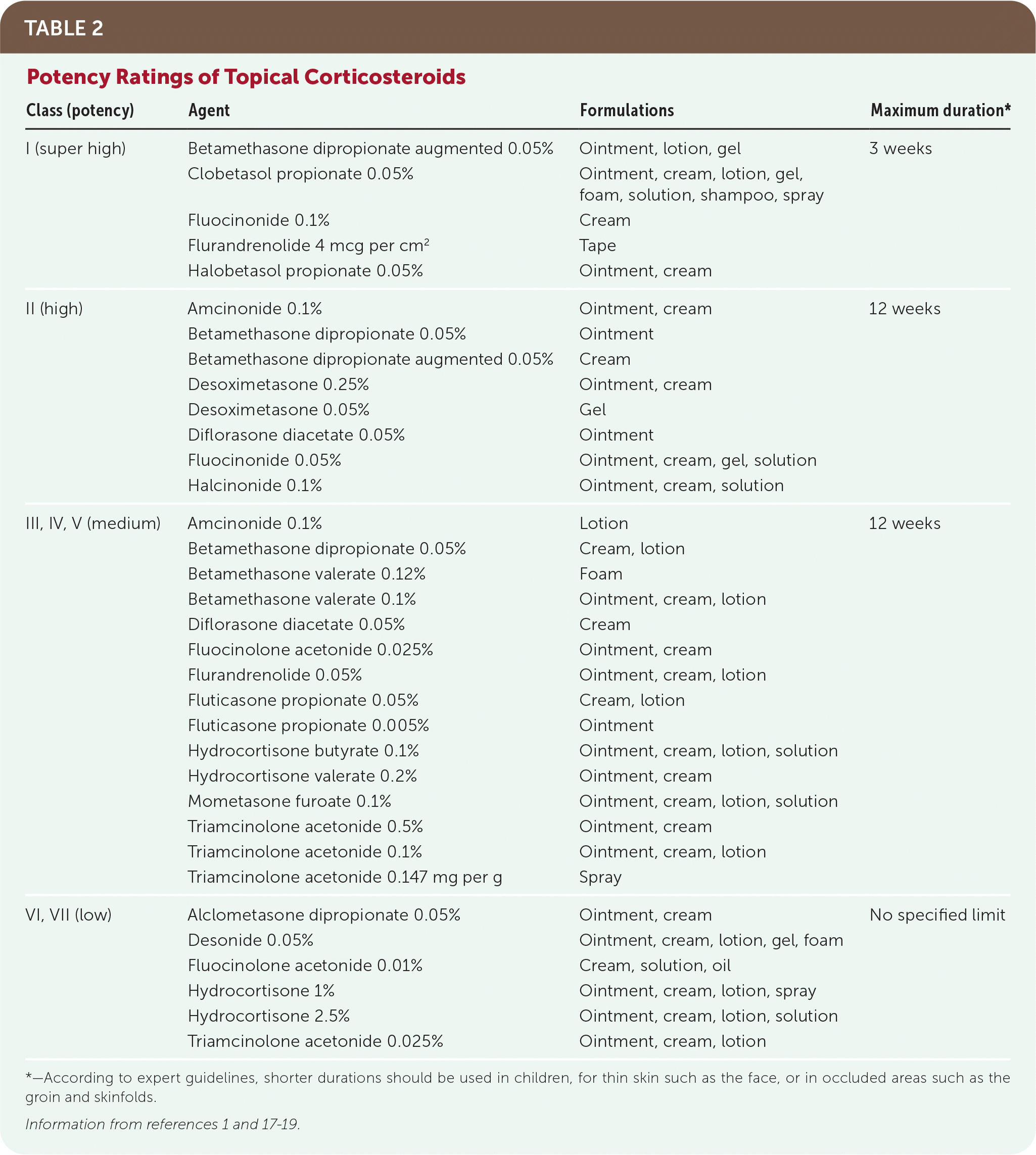
| Class (potency) | Agent | Formulations | Maximum duration* |
|---|---|---|---|
| I (super high) | Betamethasone dipropionate augmented 0.05% | Ointment, lotion, gel | 3 weeks |
| Clobetasol propionate 0.05% | Ointment, cream, lotion, gel, foam, solution, shampoo, spray | ||
| Fluocinonide 0.1% | Cream | ||
| Flurandrenolide 4 mcg per cm2 | Tape | ||
| Halobetasol propionate 0.05% | Ointment, cream | ||
| II (high) | Amcinonide 0.1% | Ointment, cream | 12 weeks |
| Betamethasone dipropionate 0.05% | Ointment | ||
| Betamethasone dipropionate augmented 0.05% | Cream | ||
| Desoximetasone 0.25% | Ointment, cream | ||
| Desoximetasone 0.05% | Gel | ||
| Diflorasone diacetate 0.05% | Ointment | ||
| Fluocinonide 0.05% | Ointment, cream, gel, solution | ||
| Halcinonide 0.1% | Ointment, cream, solution | ||
| III, IV, V (medium) | Amcinonide 0.1% | Lotion | 12 weeks |
| Betamethasone dipropionate 0.05% | Cream, lotion | ||
| Betamethasone valerate 0.12% | Foam | ||
| Betamethasone valerate 0.1% | Ointment, cream, lotion | ||
| Diflorasone diacetate 0.05% | Cream | ||
| Fluocinolone acetonide 0.025% | Ointment, cream | ||
| Flurandrenolide 0.05% | Ointment, cream, lotion | ||
| Fluticasone propionate 0.05% | Cream, lotion | ||
| Fluticasone propionate 0.005% | Ointment | ||
| Hydrocortisone butyrate 0.1% | Ointment, cream, lotion, solution | ||
| Hydrocortisone valerate 0.2% | Ointment, cream | ||
| Mometasone furoate 0.1% | Ointment, cream, lotion, solution | ||
| Triamcinolone acetonide 0.5% | Ointment, cream | ||
| Triamcinolone acetonide 0.1% | Ointment, cream, lotion | ||
| Triamcinolone acetonide 0.147 mg per g | Spray | ||
| VI, VII (low) | Alclometasone dipropionate 0.05% | Ointment, cream | No specified limit |
| Desonide 0.05% | Ointment, cream, lotion, gel, foam | ||
| Fluocinolone acetonide 0.01% | Cream, solution, oil | ||
| Hydrocortisone 1% | Ointment, cream, lotion, spray | ||
| Hydrocortisone 2.5% | Ointment, cream, lotion, solution | ||
| Triamcinolone acetonide 0.025% | Ointment, cream, lotion |
Adverse Effects
Topical corticosteroids may cause cutaneous and systemic adverse effects (Table 320). A larger area of application and higher potency of corticosteroid increases the risk of adverse effects. The use of lower-potency corticosteroids and reduction in application frequency can significantly decrease the risk of adverse effects. Thin skin (e.g., the face, intertriginous areas) and the skin of children are more susceptible to adverse effects.17
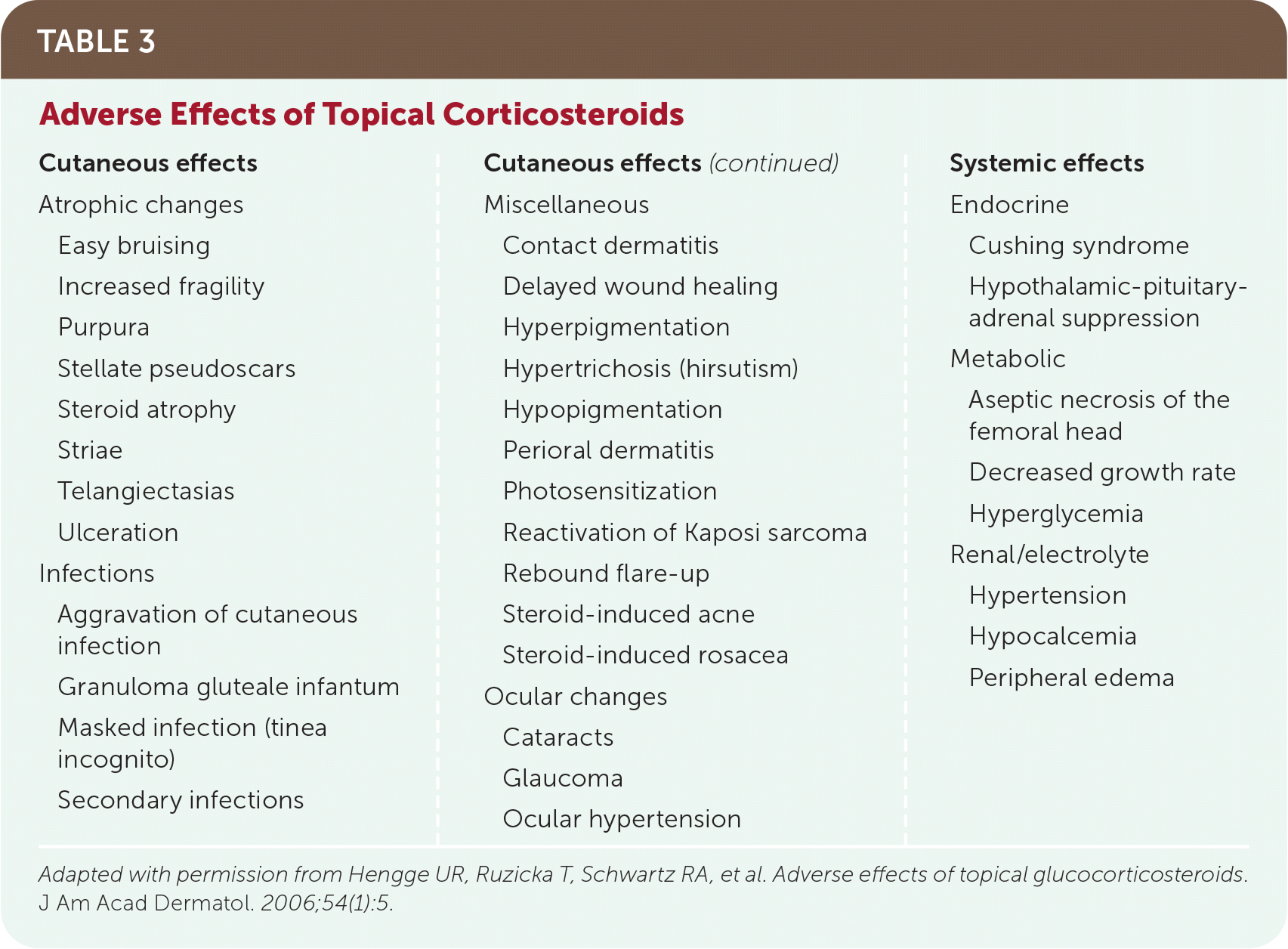
| Cutaneous effects Atrophic changes Easy bruising Increased fragility Purpura Stellate pseudoscars Steroid atrophy Striae Telangiectasias Ulceration Infections Aggravation of cutaneous infection Granuloma gluteale infantum Masked infection (tinea incognito) Secondary infections Miscellaneous Contact dermatitis Delayed wound healing Hyperpigmentation Hypertrichosis (hirsutism) Hypopigmentation Perioral dermatitis Photosensitization Reactivation of Kaposi sarcoma Rebound flare-up Steroid-induced acne Steroid-induced rosacea Ocular changes Cataracts Glaucoma Ocular hypertension Systemic effects Endocrine Cushing syndrome Hypothalamic-pituitary-adrenal suppression Metabolic Aseptic necrosis of the femoral head Decreased growth rate Hyperglycemia Renal/electrolyte Hypertension Hypocalcemia Peripheral edema |
The most common adverse effects of topical corticosteroids include atrophy, striae, rosacea, perioral dermatitis, acneiform eruption, and purpura.20 Other cutaneous adverse effects include folliculitis, periocular dermatitis, delayed wound healing, gluteal granulomas, telangiectasias and erythema, hypopigmentation, hypertrichosis, masking or aggravation of dermatophyte infection (tinea incognito), secondary infection, and contact dermatitis.17 Mucocutaneous infections are also common with topical corticosteroid use.20 The inflammatory suppression from topical corticosteroids can contribute to the prolongation, progression, and atypical presentation of cutaneous infections. Contact sensitization to topical corticosteroids can also occur but is rare. Differentiation from hypersensitivity to other components of the topical treatment is important if contact sensitization is suspected.
Systemic adverse effects that have been reported with topical corticosteroid use include cataracts, glaucoma, adrenal suppression, decreased growth rate, hypertension, hyperglycemia, and Cushing syndrome.20 Adverse effects are rare with low-potency topical corticosteroids.17 Evidence suggests that the risk of systemic adverse effects in adults is low if doses do not exceed 50 g per week, even with super-high-potency formulations.21 Children are also more susceptible to systemic adverse effects because of their increased ratio of body surface area to weight.
Patients undergoing long-term treatment with topical corticosteroids should be monitored for the development of adverse effects, particularly when used in thin skin areas or with susceptible populations such as children or older people.20
No association has been noted between the maternal use of topical corticosteroids and adverse pregnancy outcomes, including mode of delivery, congenital abnormality, preterm delivery, fetal death, or a low Apgar score.22 Topical corticosteroid use has not been associated with adverse effects in patients who are lactating.20
There is no evidence that topical corticosteroids lose effectiveness over time (tachyphylaxis).23 However, corticosteroid withdrawal (i.e., corticosteroid addiction) can occur upon cessation after prolonged use on the face and genitals, causing erythema, scaling, stinging, burning, papules, pustules, and edema.24
Formulations
Topical corticosteroids come in several formulations, each with advantages and disadvantages. The most common formulations include ointments, creams, lotions, gels, foams, and solutions.18
Ointments are petroleum-based. They are thick and lubricating and are useful for dry or hyperkeratotic lesions on smooth, nonhairy skin. Ointments are occlusive and leave a residue on the skin after application. They generally have fewer added compounds and are therefore less prone to provoke allergic reactions.25 Ointments can be less irritating to thin skin and should be avoided on intertriginous (e.g., groin, gluteal cleft, axilla) or hairy areas because they can promote maceration and folliculitis.
Creams and lotions are mixtures of water and oil. Lotions have higher water content than creams and are pourable. Lotions and creams vanish into the skin when applied. Creams are less occlusive than ointments, and lotions are less occlusive than creams. Lotions are easier to apply to hair-bearing areas. Lotions and creams commonly contain added compounds such as alcohols, which may cause irritation or allergic reactions.25 Lotions and creams can have a drying effect, which may be useful for acute exudative inflammation.
Gels are aqueous, dry rapidly, and leave little residue. Gels often sting when applied to inflamed, fissured, or eroded skin and have a drying effect. They are useful for hairy areas or when the patient wishes to avoid the greasy feeling of ointments, creams, and lotions.
Foams are dispensed from specialized containers, spread easily, and are especially useful for hair-bearing areas such as the scalp or beard. They are well-tolerated by patients but can be expensive.
Solutions and oils are liquids containing the desired corticosteroid. They spread easily and are useful for areas with dense hair such as the scalp or beard. They are not occlusive, and oils may leave a residue. Certain solutions may be delivered via a spray. Solutions often contain alcohols and may sting or burn when applied to inflamed, fissured, or eroded skin. Solutions and oils are less allergenic than lotions.25 Similar to solutions and oils, shampoos effectively deliver corticosteroids to hairy areas such as the scalp.
The choice of formulation affects the absorption of the corticosteroid; however, the differences in absorption between formulations do not likely result in a clinically meaningful change in effectiveness or risk of harm.26 Although ointments have classically been considered more potent than creams or lotions, evidence from multiple small, nonblinded, randomized trials suggests that creams, ointments, and lotions have similar levels of effectiveness.27,28 Patients typically prefer creams and lotions over ointments because they are less greasy and vanish more easily. Foams may be more preferred for all applications, particularly in areas with thick hair such as the scalp.29 The choice of formulation should be based on characteristics of the lesion, cost, and patient preference over the potential differences in strength.26–29
Duration and Frequency of Treatment
The duration of treatment depends on the strength of the corticosteroid and the condition being treated. In general, super-high-potency topical corticosteroids should be used for no more than three weeks at a time. High- and medium-potency corticosteroids should be used for no more than 12 weeks at a time. For treatments of longer duration, a potent corticosteroid may be used intermittently or transitioned to a milder corticosteroid for ongoing control. Lesions on the face, groin, and skinfolds may be treated in one- to two-week intervals. Therapy may be discontinued when the lesions resolve.17
Topical corticosteroids are usually applied once or twice per day. More frequent administration does not typically improve results and decreases patient adherence.30 The application frequency can be adjusted according to effect and patient tolerance. Topical corticosteroid penetration is enhanced by using an occlusive method such as a plastic wrap, bandages, or gloves.18,31 Penetration improves when the skin is moist; however, it is unclear whether corticosteroid application after a bath or shower produces superior results compared with application at other times.32
Prescribing and Application
A prescription for topical corticosteroids should include the medication name, concentration, formulation, directions for application, and amount. The choice of which agent, concentration, and formulation to use depends on the condition being treated, the location on the body, characteristics of the lesion, and patient factors such as age. The amount prescribed depends on the size of the area being treated, frequency, skin location, and duration of treatment.
A standard unit used for topical corticosteroid application is the fingertip unit. One fingertip unit is the amount of medication dispensed from a standard 5-mm nozzle over a distance from the tip of the index finger to the crease of the distal interphalangeal joint. This represents approximately 0.5 g of medication and should cover approximately 2% of the body surface area on an adult.33 The area of an adult patient's palm represents approximately 1% of body surface area or one-half of a fingertip unit. For example, 30 g should be sufficient for twice daily application to an area the size of two adult palms for 30 days (Table 4).34
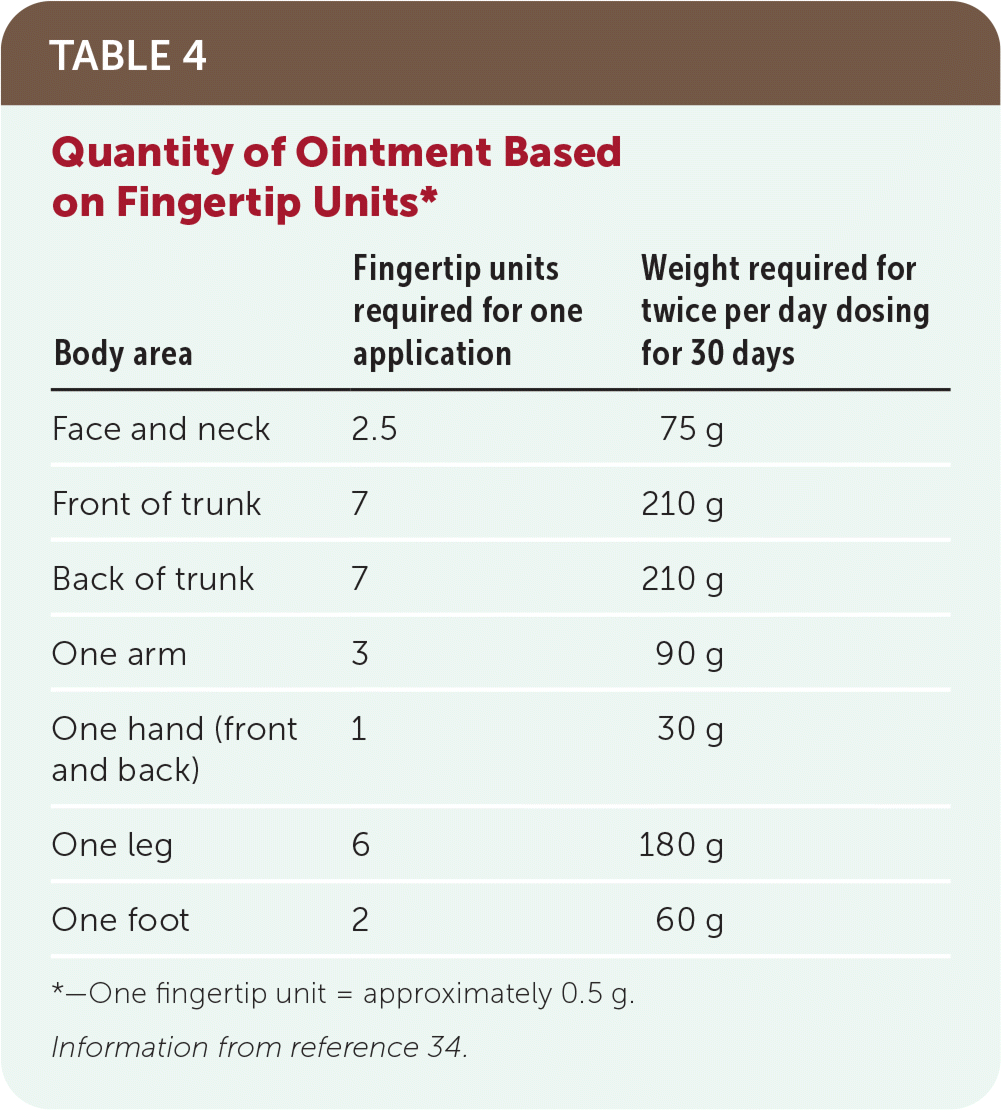
| Body area | Fingertip units required for one application | Weight required for twice per day dosing for 30 days |
|---|---|---|
| Face and neck | 2.5 | 75 g |
| Front of trunk | 7 | 210 g |
| Back of trunk | 7 | 210 g |
| One arm | 3 | 90 g |
| One hand (front and back) | 1 | 30 g |
| One leg | 6 | 180 g |
| One foot | 2 | 60 g |
Proper patient application is critical for the effectiveness of topical corticosteroids. 15,35 Patients should be taught how to apply medication using the fingertip unit method.33,34 This helps ensure that they do not apply the medication too thinly (which can result in an insufficient effect) or too thickly (which increases the risk of adverse effects).36 If a patient reports the corticosteroid is less effective than expected, clinicians should assess for difficulty with compliance and barriers to adherence. The size of an adult hand can approximate the area of application using the fingertip unit method, or the clinician may consider the approximate dose adjustment listed in Table 5.37
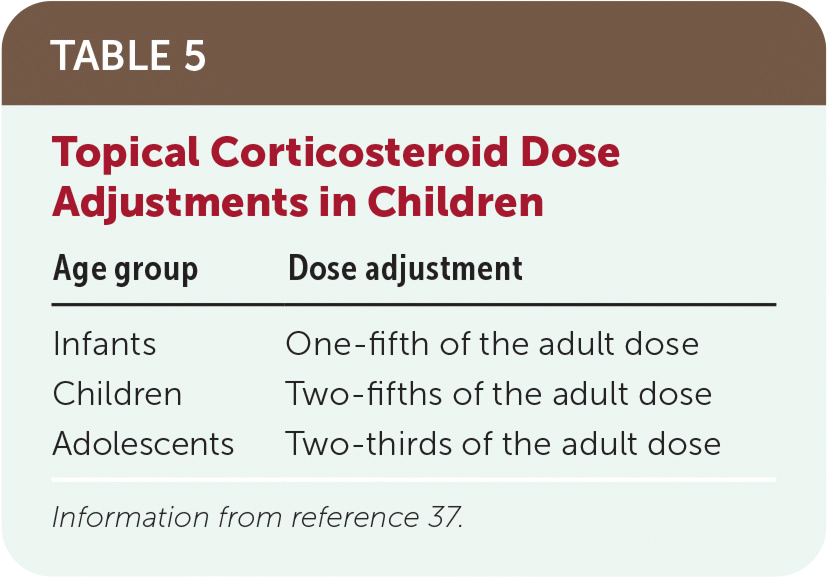
| Age group | Dose adjustment |
|---|---|
| Infants | One-fifth of the adult dose |
| Children | Two-fifths of the adult dose |
| Adolescents | Two-thirds of the adult dose |
This article updates a previous article on this topic by Ference and Last.2
Data Sources: A search of PubMed, the Cochrane database, Essential Evidence Plus, and Mayo Clinic Library services was conducted using key terms including but not limited to corticosteroid, dermatology, topical, potency, ointment, cream, foam, fingertip unit, penetration, occlusion, vasomotor, side effects, pediatric, pregnancy, breastfeeding, treatment, and other keywords identifying inflammatory skin diseases. The references from the previous American Family Physician article on this topic were reviewed and included when appropriate. Search dates: August 2020, September 2020, and February 2021.
