Am Fam Physician. 2021;103(7):430-431
Author disclosure: No relevant financial affiliations.
Digital breast tomosynthesis (DBT), or 3D mammography, is a breast imaging technique in which multiple radiographs are used to reconstruct a 3D picture. Approved by the U.S. Food and Drug Administration in 2011, DBT is purported to be more sensitive and specific than digital mammography, especially in women with dense breasts.1 Although DBT has not been universally accepted as the standard for breast cancer screening, it has supplanted digital mammography for screening in some parts of the United States; areas with higher DBT use tend to be wealthier and have larger White populations.2
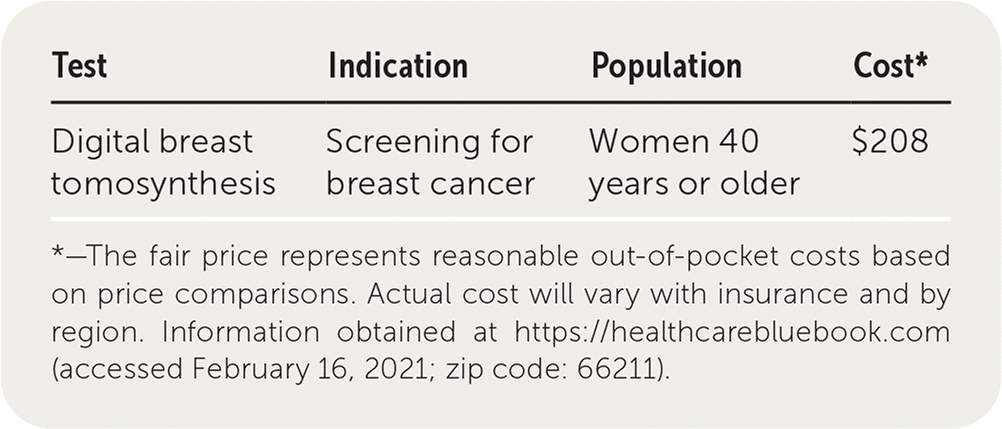
| Test | Indication | Population | Cost* |
|---|---|---|---|
| Digital breast tomosynthesis | Screening for breast cancer | Women 40 years or older | $208 |
Accuracy
In experimental studies of mastectomy specimens, DBT was superior to digital mammography in lesion detection and margin identification.3 It is not clear how this difference translates to real-life screening. The ongoing Tomosynthesis Mammographic Imaging Screening Trial compares the effectiveness of DBT and digital mammography in preventing advanced breast cancers through screening of average-risk women in the United States.4 Trial recruitment has been challenging as more hospitals adopt the newer technology. Hospitals and patients are reluctant to accept digital mammography despite the lack of evidence that DBT is superior.5 Retrospective studies suggest that when used in conjunction with digital mammography, DBT decreases recall rate and improves cancer detection in women.6
A systematic review found low-quality evidence that DBT plus digital mammography increases the overall and invasive breast cancer detection rates.7 Digital mammography alone detected 7 breast cancers per 1,000 screenings and 5 invasive breast cancers per 1,000 screenings. With the addition of DBT, detection rates increased to 10 per 1,000 screenings and 7 per 1,000 screenings, respectively. However, the review found no moderate- or high-quality evidence that DBT decreases recall rates, false-positive rates, or false-negative rates.7 In a meta-analysis of 11 studies at low risk of bias, no significant difference occurred between DBT alone and DBT plus digital mammography in cancer detection rates.8 Digital mammography is not equivalent to a 2D mammogram generated from DBT images.
Benefit
A review of seven systematic reviews from the Canadian Agency for Drugs and Technologies in Health found that DBT plus digital mammography may improve cancer detection rates (relative risk = 1.09 to 1.3) and recall rates compared with digital mammography alone. Recall rates were lowered by between 0.5 and 2 recalls per 1,000 screenings.9 3D reconstruction with DBT allows radiologists to better qualify lesions noted on digital mammography as benign, rather than calling the patient back for additional views. In the United States, however, DBT is not routinely performed with digital mammography, which may negate the benefit of a decreased recall rate.
The U.S. Preventive Services Task Force states that there is insufficient evidence to assess the benefits and harms of DBT for primary breast cancer screening in all women and in women with dense breasts.10 The American College of Radiology categorizes both digital mammography and DBT as “usually appropriate” for average- and intermediate-risk women. For high-risk women, both modalities, as well as magnetic resonance imaging with contrast, are categorized as “usually appropriate.”11 No studies have evaluated mortality as an outcome in women screened with DBT compared with digital mammography.
Harms
From a patient perspective, DBT is similar to digital mammography because both tests require that the breast be compressed between two plates and both take approximately 15 minutes to complete. The total radiation dose from DBT is almost three times that from digital mammography, but this level of radiation is still considered to be very low and safe.9 It is not known whether adding or substituting DBT for digital mammography increases the likelihood of breast cancer overdiagnosis (i.e., the detection of lesions that would never progress to symptomatic breast cancer in a normal lifetime).
Cost
Bilateral DBT costs approximately $208. In comparison, bilateral digital mammography costs about $148.12 The decreased recall rate may offset some of the total higher cost of DBT.
Although older cost-effectiveness studies showed that DBT plus digital mammography was cost-effective compared with digital mammography alone and was most cost-effective in women 40 to 49 years of age, a larger 2019 analysis showed that the overall cost of DBT remains higher than digital mammography if performed annually.13 For DBT, the benefit may be greater, and the cost difference may be lower with biennial screening.14
Bottom Line
There is currently not enough evidence to replace digital mammography with DBT for breast cancer screening in women at average or high risk. DBT offers a modestly increased cancer detection rate but comes at a higher monetary cost to the patient and the health care system, without any demonstrable mortality benefit.