
Am Fam Physician. 2021;103(10):628-629
Author disclosure: No relevant financial affiliations.
Lactic acid, citric acid, and potassium bitartrate (Phexxi) is a vaginal gel labeled for use by women as an on-demand contraception. The gel maintains vaginal pH at its physiologic acidity, creating an environment inhospitable to sperm that inhibits sperm motility.
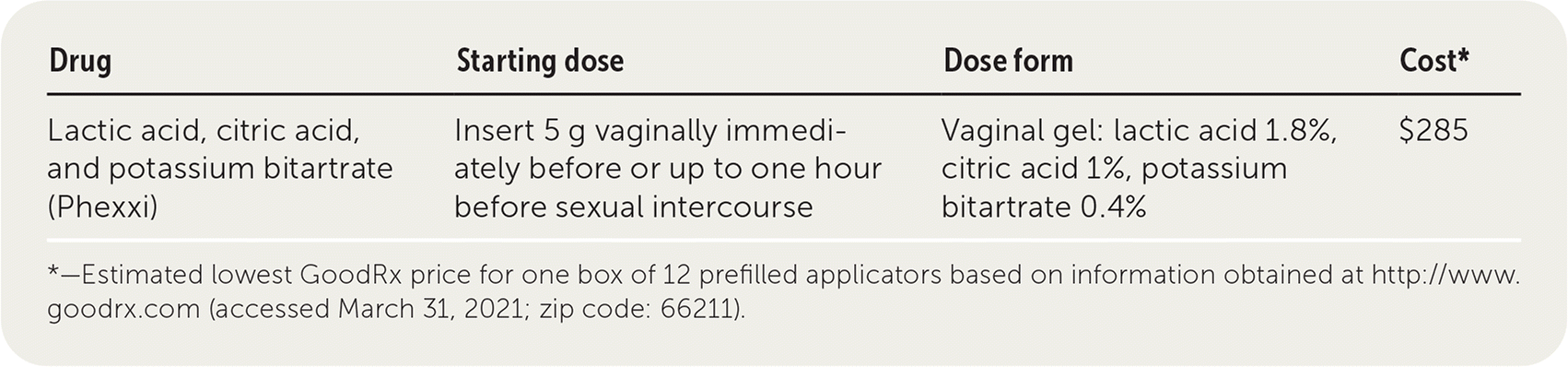
| Drug | Starting dose | Dose form | Cost* |
|---|---|---|---|
| Lactic acid, citric acid, and potassium bitartrate (Phexxi) | Insert 5 g vaginally immediately before or up to one hour before sexual intercourse | Vaginal gel: lactic acid 1.8%, citric acid 1%, potassium bitartrate 0.4% | $285 |
Safety
Phexxi is considered to be a safe contraceptive option. In two trials of 4,773 women, approximately 1% of patients experienced adverse effects, but none of the effects were shown to be caused by Phexxi.1,2 Systemic exposure to the active ingredients in the gel is minimal and is not expected to lead to safety concerns. Phexxi is not absorbed and will not cause systemic effects.3 The manufacturer recommends that Phexxi be avoided in patients with a history of recurrent urinary tract infections or urinary tract abnormalities. The water-based gel does not interact with other vaginal products like miconazole, metronidazole (Metrogel), and tioconazole.3 Phexxi is safe to use in conjunction with hormonal contraceptives, latex, polyurethane, polyisoprene condoms, and vaginal diaphragms. It should not be used with vaginal rings.3 Phexxi can be used after childbirth as soon as it is safe to resume vaginal intercourse.
Tolerability
The most common adverse effects of Phexxi are localized to the vagina and include burning sensation (18%), itching (14.5%), vulvovaginal mycotic infection (9.1%), urinary tract infection (9%), bacterial vaginosis (8.1%), and vaginal discharge (5.5%).3 More than 45% of study participants experienced at least one adverse effect, but the majority were deemed mild to moderate in severity, and only 2% to 3% of study participants dropped out because of adverse effects.1,2 These rates and types of adverse effects are similar to those that occur with nonoxynol-9, the ingredient in over-the-counter spermicidal products.2 Male partners may also experience mild reactions such as itching, burning, and pain (9.8%).3
Effectiveness
Two studies have evaluated contraceptive effectiveness over an average of six months of use. In a case series of 1,330 women 18 to 35 years of age, 13.7% of women became pregnant during six months of use.1 In an unpublished randomized controlled trial of 3,324 women 18 to 35 years of age comparing Phexxi with the spermicide nonoxynol-9, Phexxi demonstrated an 89.5% pregnancy prevention rate with typical use and 95.9% with ideal use over six months, similar to the rates with nonoxynol-9 (90.0% and 95.8%, respectively).2 Although there are no data regarding 12-month effectiveness of Phexxi, the six-month typical-use rates would likely be comparable to other on-demand methods such as condoms (87% pregnancy prevention rate with typical use over 12 months). However, pregnancy prevention rates with Phexxi are lower than with oral contraceptives (92%) and intrauterine devices or progestin implants (greater than 99%).4 Table 1 compares first-year pregnancy prevention rates of various contraceptive methods with typical and ideal use.2,4
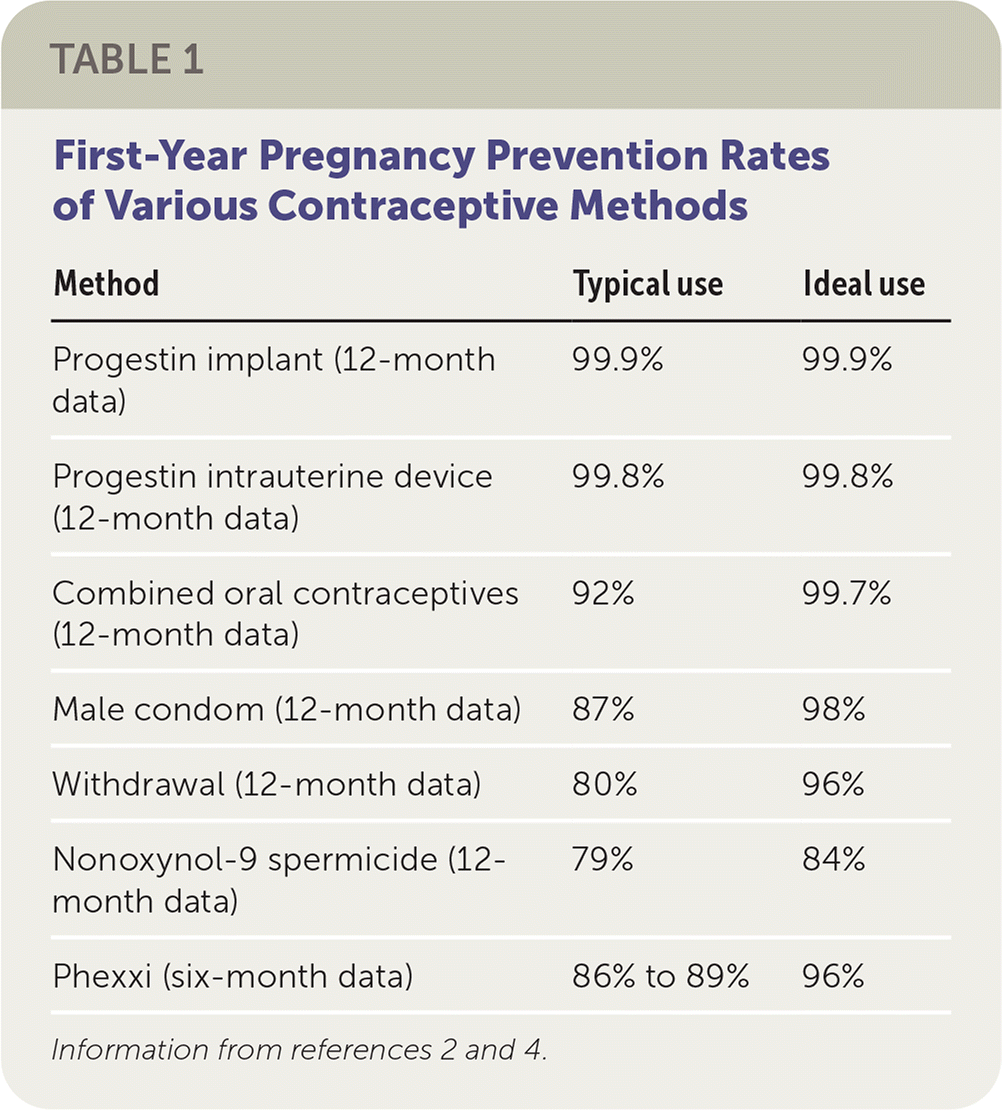
| Method | Typical use | Ideal use |
|---|---|---|
| Progestin implant (12-month data) | 99.9% | 99.9% |
| Progestin intrauterine device (12-month data) | 99.8% | 99.8% |
| Combined oral contraceptives (12-month data) | 92% | 99.7% |
| Male condom (12-month data) | 87% | 98% |
| Withdrawal (12-month data) | 80% | 96% |
| Nonoxynol-9 spermicide (12-month data) | 79% | 84% |
| Phexxi (six-month data) | 86% to 89% | 96% |
Price
One box of 12 prefilled applicators of Phexxi costs about $285, or approximately $24 for on-demand contraception per act of intercourse. One box of 10 prefilled applicators of spermicide nonoxynol-9 is $11, or about $1 per act of intercourse.5 Condoms, often available free of charge at many civic and family planning organizations, offer on-demand contraception for $0.25 to $0.50 per act of intercourse.
Simplicity
A prefilled applicator of Phexxi (5 g) must be inserted into the vagina immediately before or up to one hour before each act of vaginal intercourse. An additional 5-g dose must be used if more than one act of vaginal intercourse occurs within one hour. Phexxi can be stored at room temperature.
Bottom Line
Given its high cost, mediocre contraceptive protection, and rate of adverse effects, Phexxi may be preferred only by people desiring to avoid hormonal contraception who wish to use on-demand contraception that they control, or for whom other contraceptive options are not suited or desired.
