
Am Fam Physician. 2021;103(10):633-636
Related editorial: The KIDs List: Medications That Are Potentially Inappropriate in Children
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• The KIDs List includes a limited number of medications to avoid or use with caution in children older than one year. More medications are included for infants and neonates.
• Medications such as fluoroquinolones and antidepressants are not included because there are no effective alternatives for some indications.
• Aspirin and other salicylates should be used with caution in suspected viral respiratory illness but may be necessary for other indications.
From the AFP Editors
Adverse drug reactions are responsible for approximately 4% of admissions to children's hospitals. Up to 18% of hospitalized children experience at least one adverse drug reaction. Approximately one-half of pediatric adverse drug reactions affect children between one and 10 years of age, with the other half evenly split between infants and children older than 10 years. Because one-half of medications are not currently labeled for children, off-label prescriptions are common. The Pediatric Pharmacy Association produced a list of key potentially inappropriate drugs in pediatrics, or the KIDs List, to guide physicians who treat children.
Potentially Inappropriate Medications
The panel defined potentially inappropriate medications as medications or medication classes that generally should be avoided in children younger than 18 years because they pose an unnecessarily high risk and for which a safer alternative is available. Some medications on the list include a caveat if there is an indication for which this medication may be required.
Similar to the Beers criteria for older patients, two recommendation levels were included, avoid and use with caution. Avoid recommendations signify that most children should not receive the medication. They are either strong recommendations based on high-quality evidence or have potential adverse effects that are life-threatening or life-altering. Recommendations to use with caution signify the medication may be used, especially if there is a clear therapeutic need. These weak recommendations are based on low-quality evidence of potential adverse effects.
Medications were identified through a systematic literature review, U.S. Food and Drug Administration communications, and pharmacology databases. Included medications are commercially available in the United States, have a documented adverse effect in children that is more common or severe than that in adults, and have a safer alternative treatment. Allergic reactions, teratogenicity, and breastfeeding exposures were not considered to be adverse events.
KIDs List
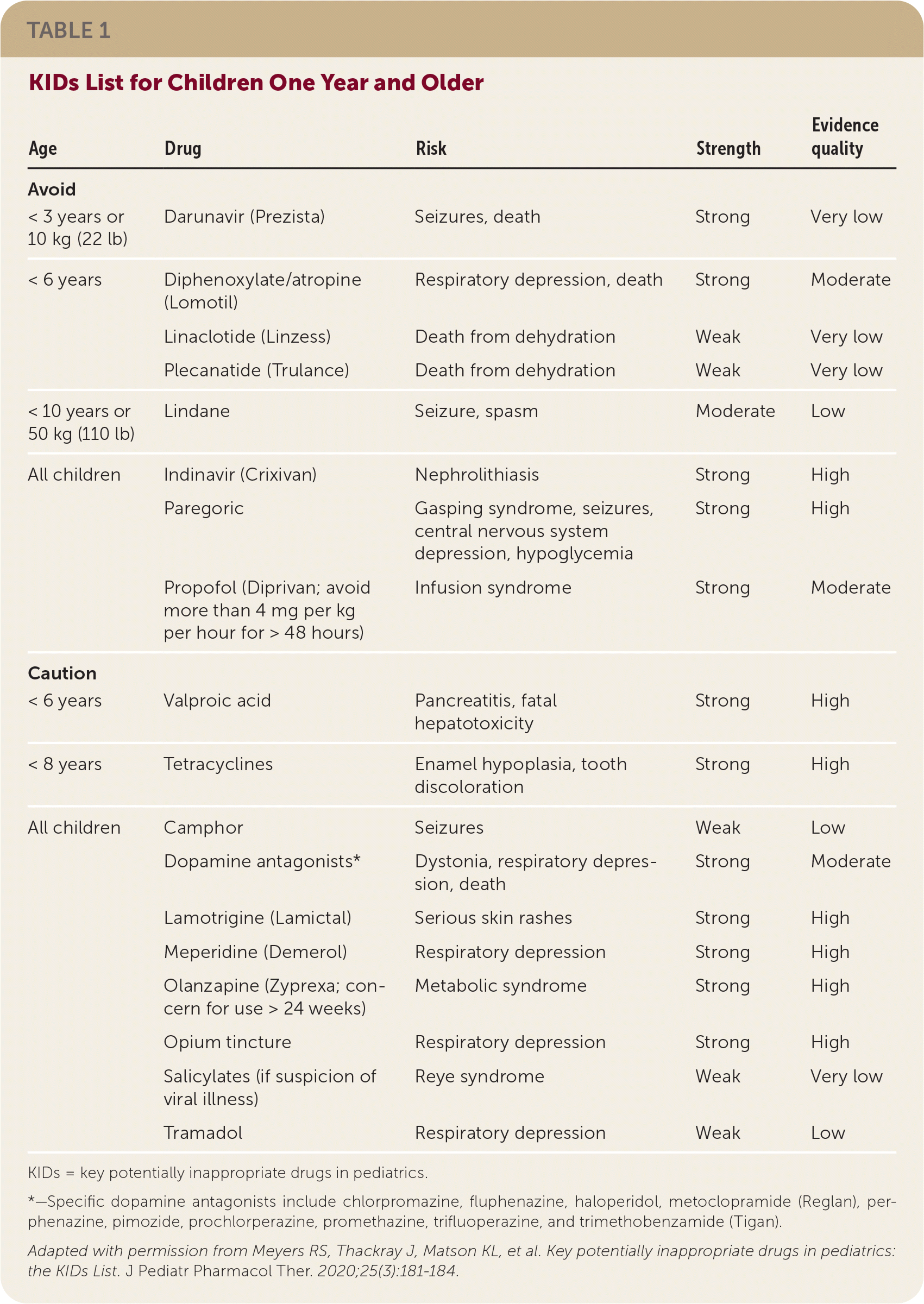
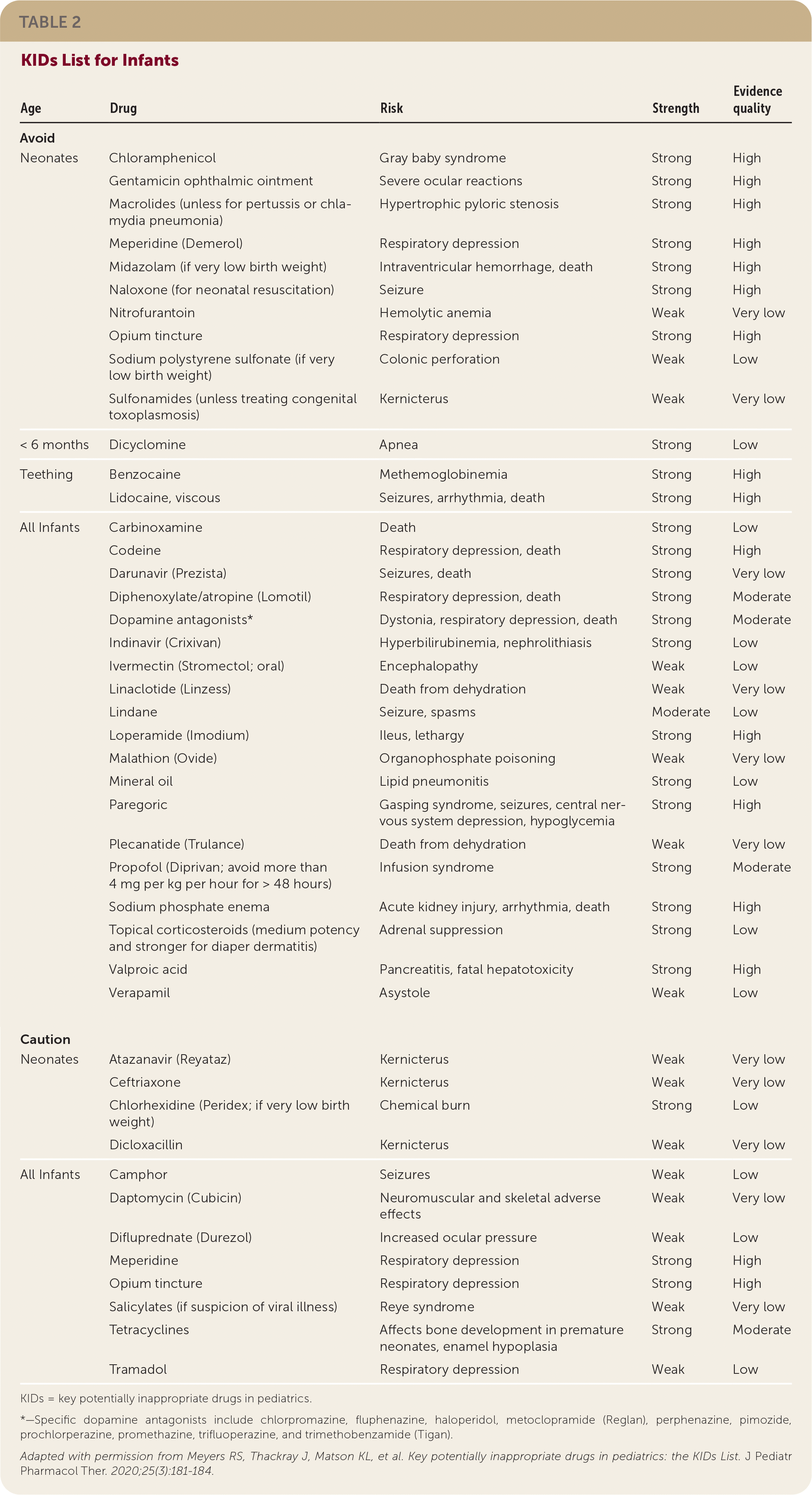
For children one year or older, the group identified eight medications or medication classes, some simply restricted by age or weight, that should be avoided and 10 that should be used with caution (Table 1). They identified more than 30 medications or medication classes to be avoided in infants and 12 to be used with caution (Table 2). Fourteen of these medication recommendations apply only to newborns. Although some of the drugs are considered by the World Health Organization to be essential medicines for children, acceptable alternatives are readily available in the United States.
| Age | Drug | Risk | Strength | Evidence quality |
|---|---|---|---|---|
| Avoid | ||||
| < 3 years or 10 kg (22 lb) | Darunavir (Prezista) | Seizures, death | Strong | Very low |
| < 6 years | Diphenoxylate/atropine (Lomotil) | Respiratory depression, death | Strong | Moderate |
| Linaclotide (Linzess) | Death from dehydration | Weak | Very low | |
| Plecanatide (Trulance) | Death from dehydration | Weak | Very low | |
| < 10 years or 50 kg (110 lb) | Lindane | Seizure, spasm | Moderate | Low |
| All children | Indinavir (Crixivan) | Nephrolithiasis | Strong | High |
| Paregoric | Gasping syndrome, seizures, central nervous system depression, hypoglycemia | Strong | High | |
| Propofol (Diprivan; avoid more than 4 mg per kg per hour for > 48 hours) | Infusion syndrome | Strong | Moderate | |
| Caution | ||||
| < 6 years | Valproic acid | Pancreatitis, fatal hepatotoxicity | Strong | High |
| < 8 years | Tetracyclines | Enamel hypoplasia, tooth discoloration | Strong | High |
| All children | Camphor | Seizures | Weak | Low |
| Dopamine antagonists* | Dystonia, respiratory depression, death | Strong | Moderate | |
| Lamotrigine (Lamictal) | Serious skin rashes | Strong | High | |
| Meperidine (Demerol) | Respiratory depression | Strong | High | |
| Olanzapine (Zyprexa; concern for use > 24 weeks) | Metabolic syndrome | Strong | High | |
| Opium tincture | Respiratory depression | Strong | High | |
| Salicylates (if suspicion of viral illness) | Reye syndrome | Weak | Very low | |
| Tramadol | Respiratory depression | Weak | Low |
| Age | Drug | Risk | Strength | Evidence quality |
|---|---|---|---|---|
| Avoid | ||||
| Neonates | Chloramphenicol | Gray baby syndrome | Strong | High |
| Gentamicin ophthalmic ointment | Severe ocular reactions | Strong | High | |
| Macrolides (unless for pertussis or chlamydia pneumonia) | Hypertrophic pyloric stenosis | Strong | High | |
| Meperidine (Demerol) | Respiratory depression | Strong | High | |
| Midazolam (if very low birth weight) | Intraventricular hemorrhage, death | Strong | High | |
| Naloxone (for neonatal resuscitation) | Seizure | Strong | High | |
| Nitrofurantoin | Hemolytic anemia | Weak | Very low | |
| Opium tincture | Respiratory depression | Strong | High | |
| Sodium polystyrene sulfonate (if very low birth weight) | Colonic perforation | Weak | Low | |
| Sulfonamides (unless treating congenital toxoplasmosis) | Kernicterus | Weak | Very low | |
| < 6 months | Dicyclomine | Apnea | Strong | Low |
| Teething | Benzocaine | Methemoglobinemia | Strong | High |
| Lidocaine, viscous | Seizures, arrhythmia, death | Strong | High | |
| All Infants | Carbinoxamine | Death | Strong | Low |
| Codeine | Respiratory depression, death | Strong | High | |
| Darunavir (Prezista) | Seizures, death | Strong | Very low | |
| Diphenoxylate/atropine (Lomotil) | Respiratory depression, death | Strong | Moderate | |
| Dopamine antagonists* | Dystonia, respiratory depression, death | Strong | Moderate | |
| Indinavir (Crixivan) | Hyperbilirubinemia, nephrolithiasis | Strong | Low | |
| Ivermectin (Stromectol; oral) | Encephalopathy | Weak | Low | |
| Linaclotide (Linzess) | Death from dehydration | Weak | Very low | |
| Lindane | Seizure, spasms | Moderate | Low | |
| Loperamide (Imodium) | Ileus, lethargy | Strong | High | |
| Malathion (Ovide) | Organophosphate poisoning | Weak | Very low | |
| Mineral oil | Lipid pneumonitis | Strong | Low | |
| Paregoric | Gasping syndrome, seizures, central nervous system depression, hypoglycemia | Strong | High | |
| Plecanatide (Trulance) | Death from dehydration | Weak | Very low | |
| Propofol (Diprivan; avoid more than 4 mg per kg per hour for > 48 hours) | Infusion syndrome | Strong | Moderate | |
| Sodium phosphate enema | Acute kidney injury, arrhythmia, death | Strong | High | |
| Topical corticosteroids (medium potency and stronger for diaper dermatitis) | Adrenal suppression | Strong | Low | |
| Valproic acid | Pancreatitis, fatal hepatotoxicity | Strong | High | |
| Verapamil | Asystole | Weak | Low | |
| Caution | ||||
| Neonates | Atazanavir (Reyataz) | Kernicterus | Weak | Very low |
| Ceftriaxone | Kernicterus | Weak | Very low | |
| Chlorhexidine (Peridex; if very low birth weight) | Chemical burn | Strong | Low | |
| Dicloxacillin | Kernicterus | Weak | Very low | |
| All Infants | Camphor | Seizures | Weak | Low |
| Daptomycin (Cubicin) | Neuromuscular and skeletal adverse effects | Weak | Very low | |
| Difluprednate (Durezol) | Increased ocular pressure | Weak | Low | |
| Meperidine | Respiratory depression | Strong | High | |
| Opium tincture | Respiratory depression | Strong | High | |
| Salicylates (if suspicion of viral illness) | Reye syndrome | Weak | Very low | |
| Tetracyclines | Affects bone development in premature neonates, enamel hypoplasia | Strong | Moderate | |
| Tramadol | Respiratory depression | Weak | Low |
The list does not include over-the-counter cold medicines, which are generally safe (although not necessarily effective) at labeled doses. The group did not include fluoroquinolone antibiotics or antidepressant medications, even though they have been linked to suicidal ideation and suicidality, because the evidence of harm is weak and alternatives are not available.
The group does not suggest avoiding aspirin and other salicylate-containing medicines (e.g., bismuth subsalicylate) even though they have been linked to Reye syndrome. The group cites a weak association and notes important uses such as Kawasaki disease. Aspirin should be used with caution in children with suspected viral illness such as influenza or varicella.
Editor's Note: This is the first effort to make a list of medications to avoid in children that is similar to the lists for older adults such as the Beers and START/STOPP criteria. Fortunately, this group came up with few medications to avoid that are commonly used in infants and children. However, it is a good resource when considering medications not usually used for common childhood concerns. Similar to the Beers criteria, this list may be best suited for the electronic health record to trigger warnings when prescribing these medications.—Michael J. Arnold, MD, Contributing Editor
The views expressed are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.
Guideline source: Pediatric Pharmacy Association
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: J Pediatr Pharmacol Ther. 2020;25(3):175–191
Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7134587/
