
Am Fam Physician. 2021;103(11):656-657
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: Patients being actively managed in the third stage of labor who also have postpartum hemorrhage
Efficacy End Points: Composite outcome of maternal death or severe morbidity (primary); need for blood transfusion (secondary)
Harm End Points: Fever, vomiting (secondary)
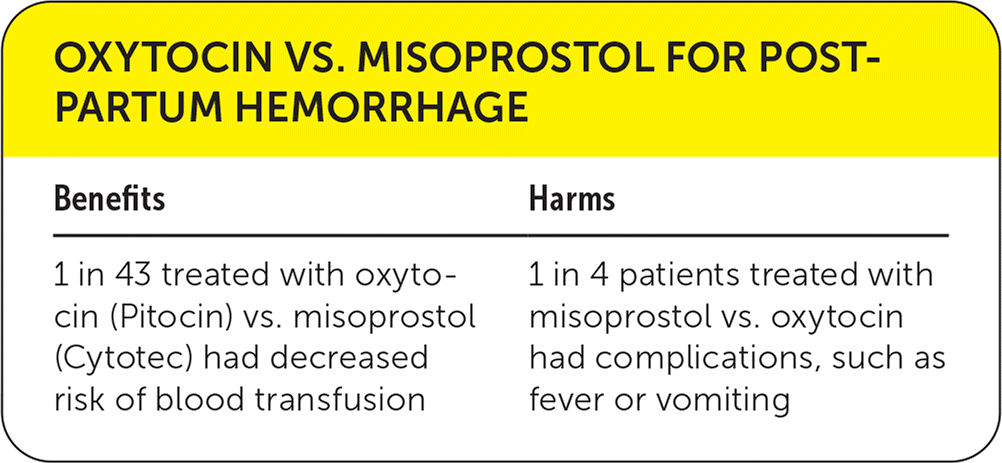
| Benefits | Harms |
|---|---|
| 1 in 43 treated with oxytocin (Pitocin) vs. misoprostol (Cytotec) had decreased risk of blood transfusion | 1 in 4 patients treated with misoprostol vs. oxytocin had complications, such as fever or vomiting |
Narrative: Postpartum hemorrhage (i.e., more than 500 mL of maternal blood loss) is the leading cause of postpartum maternal mortality worldwide and a significant contributing cause of neonatal morbidity and mortality.1 Uterine atony is the most common cause of postpartum hemorrhage. Although the World Health Organization recommends using prophylactic uterotonics for all delivering patients, postpartum hemorrhage still occurs in up to 15% of births worldwide. There is consensus among maternal health organizations that uterotonics should be considered as first-line therapy for postpartum hemorrhage.2 However, there is no consensus on a recommended first-line agent.
When readily available, oxytocin (Pitocin) is commonly used as the first-line uterotonic. Oxytocin monotherapy decreases the need for blood transfusion and has fewer adverse effects than misoprostol (Cytotec) monotherapy or misoprostol plus oxytocin.3 The effect of oxytocin therapy on mortality or severe maternal morbidity is unclear. A pairwise meta-analysis of two trials, including 1,787 participants, suggests that using misoprostol as first-line therapy increases the risk of blood transfusion compared with oxytocin (risk ratio = 1.47; 95% CI, 1.02 to 2.14).3
This Cochrane review compared uterotonics to treat postpartum hemorrhage and involved seven studies and 3,738 total patients.3 All included studies were two-arm clinical trials in hospital settings in Argentina, Burkina Faso, Ecuador, Egypt, Gambia, Pakistan, South Africa, Thailand, Turkey, or Vietnam. Of the participants, 98% delivered vaginally. Prophylactic uterotonics were used in all but one trial (978 participants).4
One trial with 809 participants reported a composite outcome of death or severe morbidity, defined as hysterectomy, transfer to higher care, organ dysfunction, coagulopathy, or shock. In this trial, there were two deaths (one in each treatment arm) and six hysterectomies (four in the misoprostol group, two in the oxytocin group), but the results were not clinically significant.5 Two studies with 1,787 total participants reported transfusion data. Out of 895 patients receiving misoprostol, 65 needed a blood transfusion, and out of 892 patients receiving oxytocin, 44 needed a blood transfusion (absolute risk reduction = 2.3%; 95% CI, 0.11 to 4.55).4,5 Four studies with 1,877 total participants compared oxytocin monotherapy with oxytocin plus misoprostol. There was no discernible difference between the two groups (risk reduction = 0.95; 95% CI, 0.77 to 1.17).6–9
Fever and vomiting were the most reported adverse effects across trials and occurred significantly more often in the misoprostol treatment arms. Out of 1,821 patients who received misoprostol, 650 developed a fever, compared with 206 out of 1,840 patients not taking misoprostol (absolute risk increase = 24.5%; 95% CI, 20.78 to 28.1). Vomiting was more common in the misoprostol arms but to a less significant extent (number needed to harm = 36; 95% CI, 21.9 to 81).6–9
Caveats: The individual study quality in this Cochrane review was heterogeneous, with most available evidence rated as low certainty. Variable postpartum hemorrhage definitions, estimated blood volume loss in some trials, and different quantifiable inclusion thresholds (e.g., 700 mL vs. 500 mL) reduced the evidence certainty.4,5 Selective reporting was a concern in four of the studies, with no reported outcomes specified in published protocols, and two of the studies registered outcomes only retrospectively. Locations of clinical trials within the developing world and inadequate descriptions of delivery team training also limit generalizability to U.S. populations.
Variability in prophylactic and treatment doses of oxytocin and in delivery timing also limits external validity. Misoprostol dosing methods varied, including sublingual, rectal, oral, and combination doses from 400 mcg to 1,000 mcg. Although several patient-oriented outcomes were reported in this review, the included trials did not consider numerous measures of maternal well-being; breastfeeding rates; or markers of morbidity, such as hypotension or need for delayed transfusion.
Storage and administration requirements of oxytocin preclude stronger recommendations for its use as a first-line agent, especially in the developing world or nonhospital settings, but it seems to offer a compelling advantage when it is readily available.
The World Health Organization advocates for using intravenous oxytocin as the preferred uterotonic in postpartum hemorrhage treatment. No other major maternal health organization specifically recommends a first-line therapeutic uterotonic.2,10–12 National Institute for Health and Care Excellence guidelines support the use of oxytocin or ergometrine (not available in the United States) as a first-line uterotonic.13 These organizations advocate using oxytocin in some form as a potential therapeutic uterotonic in postpartum hemorrhage, although strategies for dosing and administration vary.
Conclusion: We assign a color rating of yellow. Oxytocin therapy decreases the chances of a blood transfusion compared with misoprostol and has fewer adverse effects. Weak patient-oriented outcome data and challenges in the storage and administration of oxytocin prevent it from being the universally preferred uterotonic for treating postpartum hemorrhage.
The views expressed are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Uniformed Services University of the Health Sciences, Fort Belvoir Community Hospital, the Department of Defense, or the U.S. government.
