
Am Fam Physician. 2021;103(12):online
Related Putting Prevention into Practice: Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Persons
As published by the USPSTF.
Summary of Recommendations
The USPSTF recommends that clinicians ask all adults about tobacco use, advise them to stop using tobacco, and provide behavioral interventions and U.S. Food and Drug Administration (FDA)–approved pharmacotherapy for cessation to nonpregnant adults who use tobacco (Table 1). A recommendation.
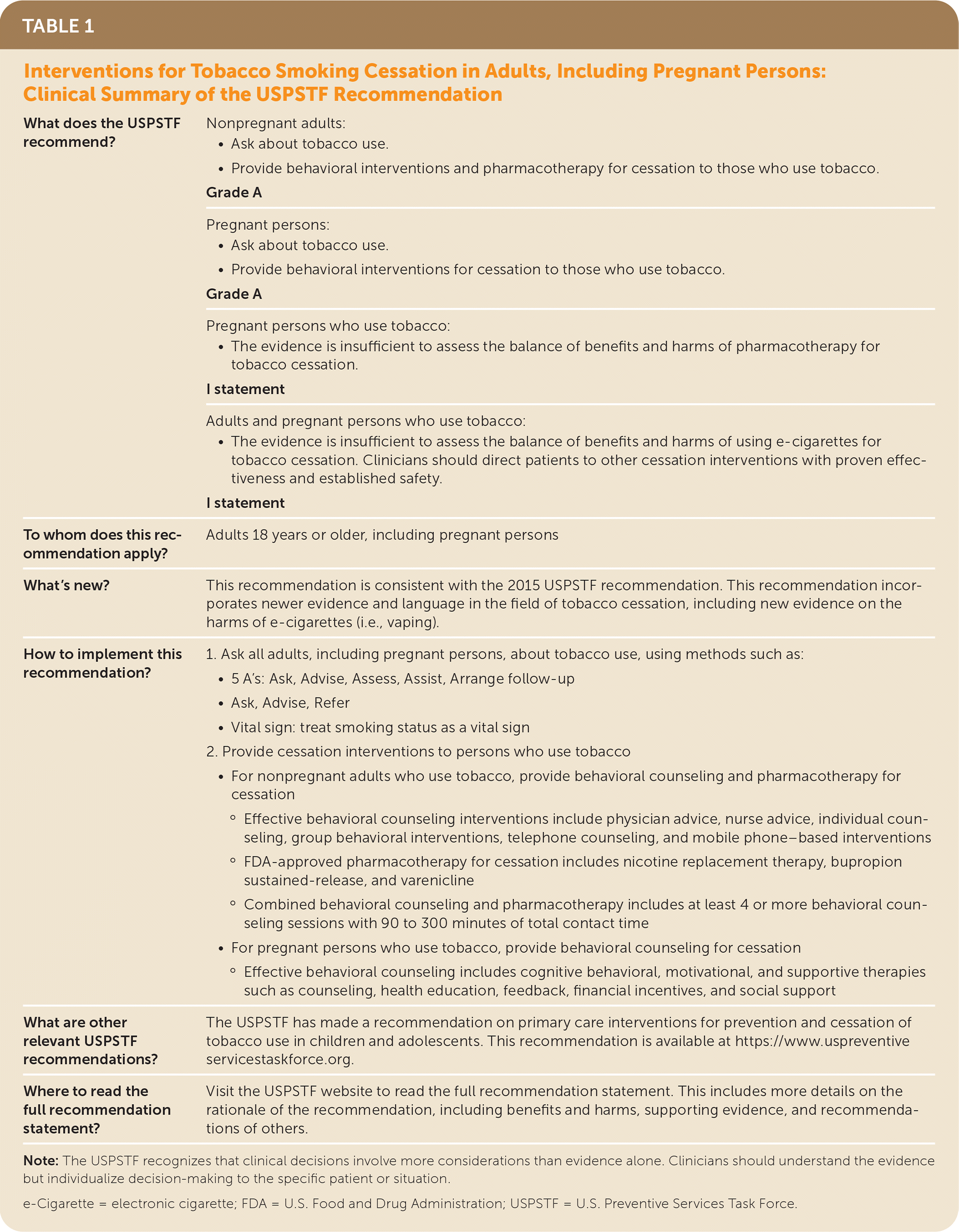
| What does the USPSTF recommend? | Nonpregnant adults:
Grade A |
Pregnant persons:
Grade A | |
Pregnant persons who use tobacco:
I statement | |
Adults and pregnant persons who use tobacco:
I statement | |
| To whom does this recommendation apply? | Adults 18 years or older, including pregnant persons |
| What's new? | This recommendation is consistent with the 2015 USPSTF recommendation. This recommendation incorporates newer evidence and language in the field of tobacco cessation, including new evidence on the harms of e-cigarettes (i.e., vaping). |
| How to implement this recommendation? |
|
| What are other relevant USPSTF recommendations? | The USPSTF has made a recommendation on primary care interventions for prevention and cessation of tobacco use in children and adolescents. This recommendation is available at https://www.uspreventiveservicestaskforce.org. |
| Where to read the full recommendation statement? | Visit the USPSTF website to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms, supporting evidence, and recommendations of others. |
The USPSTF recommends that clinicians ask all pregnant persons about tobacco use, advise them to stop using tobacco, and provide behavioral interventions for cessation to pregnant persons who use tobacco (Table 1). A recommendation.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of pharmacotherapy interventions for tobacco cessation in pregnant persons (Table 1). I statement.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of electronic cigarettes (e-cigarettes) for tobacco cessation in adults, including pregnant persons. The USPSTF recommends that clinicians direct patients who use tobacco to other tobacco cessation interventions with proven effectiveness and established safety (Table 1). I statement.
See the Practice Considerations section for more information on recommended behavioral interventions and pharmacotherapy and for suggestions for practice regarding the I statements.
Introduction
Tobacco use is the leading preventable cause of disease, disability, and death in the United States. In 2014, it was estimated that 480,000 deaths annually are attributed to cigarette smoking, including secondhand smoke.1 Smoking during pregnancy can increase the risk for miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, placental abruption, and complications in the offspring, including sudden infant death syndrome and impaired lung function in childhood.1–4 In 2019 (the most recent data currently available), an estimated 50.6 million U.S. adults (20.8% of the adult population) used tobacco; 14.0% of the U.S. adult population currently smoked cigarettes; and 4.5% of the U.S. adult population used e-cigarettes.5 According to data from the National Vital Statistics System, in 2016, 7.2% of women who gave birth smoked cigarettes during pregnancy.6 There are disparities in smoking behaviors associated with certain sociodemographic factors: Smoking rates are particularly high in non-Hispanic American Indian/Alaska Native persons; lesbian, gay, or bisexual adults; adults whose highest level of educational attainment is a General Educational Development certificate; persons who are uninsured and those with Medicaid; adults with a disability; and persons with mild, moderate, or severe generalized anxiety symptoms.5 According to the 2015 National Health Interview Survey, which reported responses from 33,672 adults, 68% of adults who smoked reported that they wanted to stop smoking, and 55% attempted quitting in the past year7; only 7% reported having recently quit smoking, and 31% reported having used cessation counseling, medication, or both when trying to quit.7
USPSTF Assessment of Magnitude of Net Benefit
The USPSTF concludes with high certainty that the net benefit of behavioral interventions and FDA-approved pharmacotherapy for tobacco smoking cessation, alone or combined, in nonpregnant adults who smoke is substantial.
The USPSTF concludes with high certainty that the net benefit of behavioral interventions for tobacco smoking cessation on perinatal outcomes and smoking cessation in pregnant persons is substantial.
The USPSTF concludes that the evidence on pharmacotherapy interventions for tobacco smoking cessation in pregnant persons is insufficient because few studies are available, and the balance of benefits and harms cannot be determined.
The USPSTF concludes that the evidence on the use of e-cigarettes for tobacco smoking cessation in adults, including pregnant persons, is insufficient, and the balance of benefits and harms cannot be determined. The USPSTF has identified the lack of well-designed, randomized clinical trials on e-cigarettes that report smoking abstinence or adverse events as a critical gap in the evidence.

| Rationale | Nonpregnant adults | Pregnant persons |
|---|---|---|
| Benefits of intervention |
|
|
| Harms of intervention |
|
|
| USPSTF assessment |
|
|
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to adults 18 years or older, including pregnant persons. The USPSTF has issued a separate recommendation statement on primary care interventions for the prevention and cessation of tobacco use in children and adolescents.9
DEFINITIONS
Key definitions related to tobacco use are reported in Table 3.10,11 Although tobacco use refers broadly to the use of any tobacco product, cigarette smoking has historically been the most prevalent form of tobacco use in the United States, and most of the evidence surrounding cessation of tobacco products relates to quitting combustible cigarette smoking. Thus, the current USPSTF recommendations focus on interventions for tobacco smoking cessation. Additionally, although e-cigarettes are considered a tobacco product that should also be the focus of tobacco prevention and cessation efforts, for this recommendation statement, the evidence on e-cigarettes as a potential cessation aid for cigarette smoking was also evaluated.
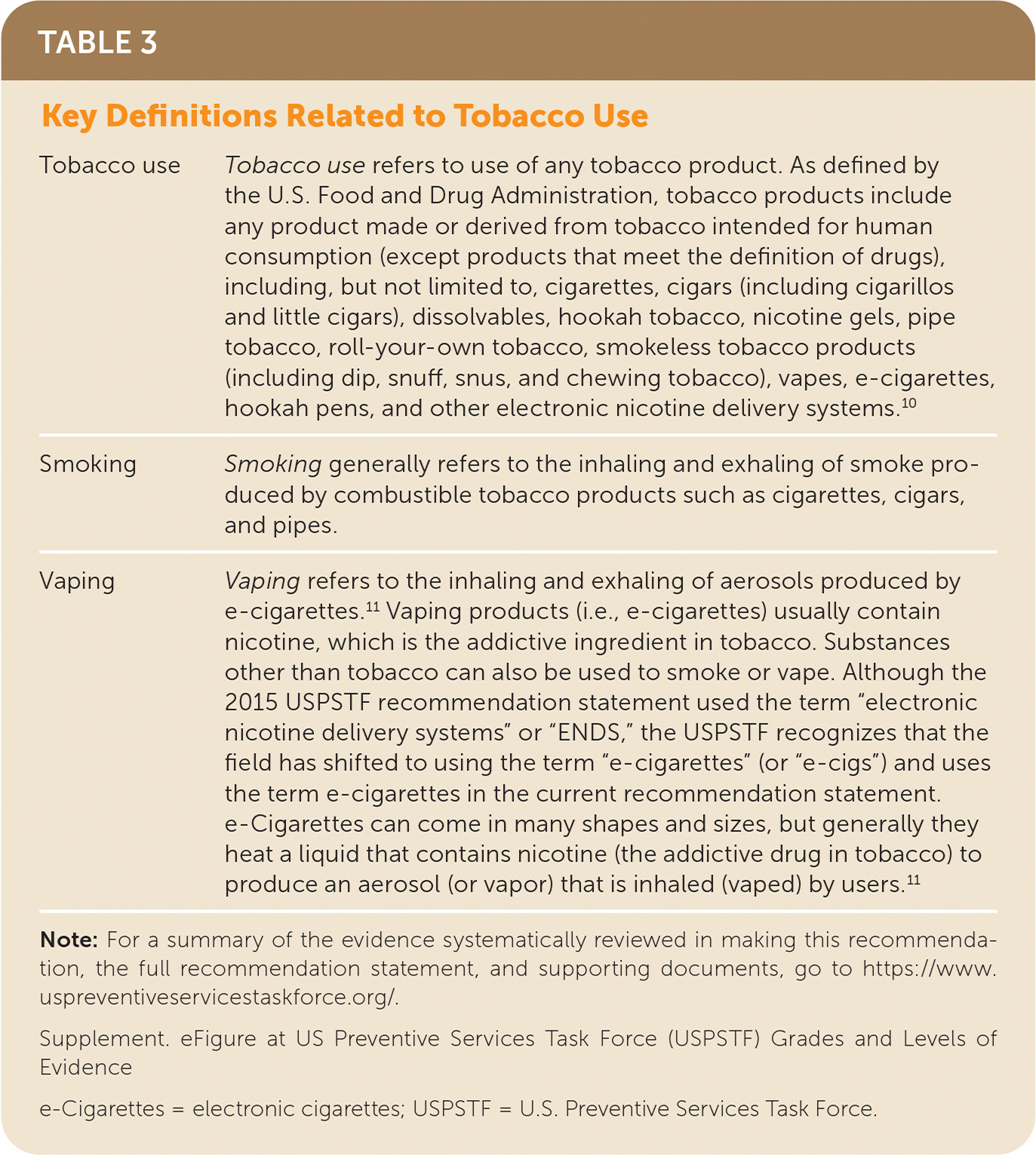
| Tobacco use | Tobacco use refers to use of any tobacco product. As defined by the U.S. Food and Drug Administration, tobacco products include any product made or derived from tobacco intended for human consumption (except products that meet the definition of drugs), including, but not limited to, cigarettes, cigars (including cigarillos and little cigars), dissolvables, hookah tobacco, nicotine gels, pipe tobacco, roll-your-own tobacco, smokeless tobacco products (including dip, snuff, snus, and chewing tobacco), vapes, e-cigarettes, hookah pens, and other electronic nicotine delivery systems.10 |
| Smoking | Smoking generally refers to the inhaling and exhaling of smoke produced by combustible tobacco products such as cigarettes, cigars, and pipes. |
| Vaping | Vaping refers to the inhaling and exhaling of aerosols produced by e-cigarettes.11 Vaping products (i.e., e-cigarettes) usually contain nicotine, which is the addictive ingredient in tobacco. Substances other than tobacco can also be used to smoke or vape. Although the 2015 USPSTF recommendation statement used the term “electronic nicotine delivery systems” or “ENDS,” the USPSTF recognizes that the field has shifted to using the term “e-cigarettes” (or “e-cigs”) and uses the term e-cigarettes in the current recommendation statement. e-Cigarettes can come in many shapes and sizes, but generally they heat a liquid that contains nicotine (the addictive drug in tobacco) to produce an aerosol (or vapor) that is inhaled (vaped) by users.11 |
ASSESSMENT OF TOBACCO USE
All patients should be asked about their tobacco use, whether or not risk factors for use are present, and encouraged to stop using tobacco. When smoking is identified, all patients should be provided interventions to quit smoking. Higher smoking prevalence has been observed in men; persons younger than 65 years; non-Hispanic American Indian/Alaska Native persons; persons who are lesbian, gay, or bisexual; persons whose highest level of educational attainment is a General Educational Development certificate; persons with an annual household income less than $35,000; persons with a disability; and persons with mild, moderate, or severe anxiety symptoms.5
Common approaches for clinicians to assess patients' tobacco use include the following.
The 5 A's: (1) ask about tobacco use; (2) advise to quit through clear, personalized messages; (3) assess willingness to quit; (4) assist in quitting; and (5) arrange follow-up and support.12
“Ask, Advise, Refer,” which encourages clinicians to ask patients about tobacco use, advise them to quit, and refer them to telephone quit lines, other evidence-based cessation interventions, or both.12
Vital sign: Treating smoking status as a vital sign and recording smoking status at every health visit are also frequently used to assess smoking status.12
Because many pregnant persons who smoke do not report it, using multiple choice questions to assess smoking status in this group may improve disclosure.12
INTERVENTIONS FOR TOBACCO CESSATION AND IMPLEMENTATION CONSIDERATIONS
Combined Behavioral Counseling Interventions and Pharmacotherapy. Combining behavioral and pharmacotherapy interventions has been shown to increase tobacco smoking cessation rates compared with either usual care/brief cessation interventions alone or pharmacotherapy alone.13 Most combination interventions include behavioral counseling involving several sessions (≥ 4), with planned total contact time usually ranging from 90 to 300 minutes.13 The largest effect was found in interventions that provided 8 or more sessions, although the difference in effect among the number of sessions was not significant.13
Behavioral Counseling Interventions. Many behavioral counseling interventions are available to increase tobacco smoking cessation in adults. These interventions can be delivered in the primary care setting or can be referred to community settings with feedback to the primary care clinician. Effective behavioral interventions include physician advice, nurse advice, individual counseling with a cessation specialist, group behavioral interventions, telephone counseling, and mobile phone–based interventions.13 Behavioral counseling interventions used in studies typically targeted individuals who were motivated to quit tobacco smoking.13 For additional information about behavioral counseling interventions in nonpregnant adults, see Table 4.12,13,15–45
| Intervention type | ||||
|---|---|---|---|---|
| Physician or nurse advice | Individual or group-based counseling | Telephone and mobile phone–based interventions | Psychosocial intervention in pregnant persons | |
| Intervention recipient | Adult smokers motivated to quit | Adult smokers, regardless of motivation to quit |
| Pregnant smokers |
| Behavior change goals and techniques | Specific advice varied but generally included a verbal stop smoking message Most often, advice was given along with print materials, additional advice from health care staff, or a referral to a cessation clinic | Typically included review of smoking history and motivation to quit, help in the identification of high-risk situations and the generation for problem solving strategies, and nonspecific support and encouragement Many group-based sessions included cognitive behavioral therapy. Initial sessions focused on discussion of motivation for quitting, health benefits, and strategies for planning a quit attempt | Telephone counseling and mobile phone–based interventions were generally tailored to participants' smoking history and readiness to quit and focused on increasing motivation and likelihood of quitting | Cognitive behavioral, motivational, and supportive therapies that include counseling, health education, feedback, financial incentives, and social support |
| Intervention intensity | Often a single session lasting less than 20 minutes (with or without print materials) plus up to 1 follow-up visit between 1 week and 3 months later | Often 1 face-to-face session with follow-up over 1 week to 4 months later Individual-based counseling given during 1 face-to-face session with multiple follow-up sessions in person or via telephone Group-based counseling delivered over 6 to 8 sessions | Varied from 2 weeks to 1 year, with most taking place over 3 to 4 months Telephone counseling: 1 to 12 calls 10 to 20 minutes per call, although the first calls were often longer Occurred during scheduled telephone calls that began after smokers had first called a smoking quit line Mobile phone–based: Fewer than 2 messages per day every day over the course of the intervention Used text messaging | Recruited during first prenatal visit or during second-trimester visit and continued through late pregnancy Frequency and intensity varied. Counseling ranged from a single session < 5 minutes to several sessions up to 4 hours per session and has been increasing over time |
| Interventionist | Physicians (e.g., general practitioners, family practice) or nursing staff | Smoking cessation specialists, often with backgrounds in social work, psychology, psychiatry, health education, and nursing | Telephone counseling provided by professional counselors or trained health care professionals Text messages were developed and administered through computer expert–generated systems | Varied |
| Practice settings | Primary care or hospital settings | Hospital or smoking cessation clinic settings | Virtual via telephone or mobile phone; a few studies provided face-to-face support | Women's health clinic or smoking cessation clinic |
| Examples of interventions and materials used in studies†,‡ | Intervention: Morgan, et al.,15 1996 Material used: Clear Horizons: A Quit Smoking Guide Especially for Those 50 and Over (Orleans, et al.,16 1989) Intervention: Canga, et al.,17 2000 Materials used: Based on How to Help Your Patients Stop Smoking: A National Cancer Institute Manual for Physicians18 and the orientation of the Mayo Nicotine Dependence Center19 | Intervention: Weissfeld, et al.,20 1991 Material used: Clearing the Air: How to Quit Smoking and Quit for Keeps (National Cancer Institute,21 1987) Other interventions: Fiore, et al.,22 2004 Glasgow, et al.,23 2000 | Intervention: Bock, et al.,24 2013 Material used: American Lung Association guide (Strecher, et al.,25 1989) Intervention: Orleans, et al.,26 1991 Material used: A Lifetime of Freedom from Smoking (American Lung Association,27 1980) Intervention: McBride, et al.,28 1999 Material used: Clearing The Air: Quit Smoking Today (National Cancer Institute, 2008 [https://www.cancer.gov/publications/patient-education/clearing-the-air]) Other interventions: Curry, et al.,29 1995; Ellerbeck, et al.,30 2009; McClure, et al.,31 2005 | Intervention: Rigotti, et al.,32 2006 Material used: Solomon and Quinn,33 2004 Intervention: Windsor, et al.,34 2011 Materials used: Ask-Advise-Assess-Arrange SCRIPT,12,35–37 including a video,38 guide to quit smoking,39 and a ≤ 10-minute counseling session40 Other interventions: Bullock, et al.,41 2009; Lee, et al.,42 2015; Pollak, et al.,43 2013; Stotts, et al.,44 2009 |
| Demonstrated benefit§ | Increases the rate of smoking cessation at 6 months or more Physician advice: 1.76 (95% CI, 1.58 to 1.96). Some evidence suggests that providing additional follow-up is more effective Nurse advice: 1.29 (95% CI, 1.21 to 1.38) | Increases the rate of smoking cessation at 6 months or more Individual counseling: RR, 1.48 (95% CI, 1.34 to 1.64) Group-based therapy: RR, 1.88 (95% CI, 1.52 to 2.33) | Increases the rate of smoking cessation at 6 months or more Telephone counseling (provided after smoker calls quit line): RR, 1.38 (95% CI, 1.19 to 1.61) Telephone counseling (other settings): RR, 1.25 (95% CI, 1.15 to 1.35) Mobile phone–based interventions: RR, 1.54 (95% CI, 1.19 to 2.00) Some evidence that interventions were more effective for smokers who were motivated to quit | Increases smoking cessation in late pregnancy: RR, 1.35 (95% CI, 1.23 to 1.48) Interventions more effective when counseling was more intensive, augmented with messages and self-help materials tailored for pregnant women, and included messages about the effects of smoking on both maternal and fetal health with strong advice to quit as soon as possible Health education, without counseling, was not effective |
Pharmacotherapy. The current pharmacotherapy interventions approved by the FDA for the treatment of tobacco smoking dependence in adults are nicotine replacement therapy (NRT; including nicotine transdermal patches, lozenges, gum, inhalers, or nasal spray), bupropion hydrochloride sustained-release, and varenicline.46 All 3 types of pharmacotherapy increase tobacco smoking cessation rates. Using a combination of NRT products (in particular, combining short-acting plus long-acting forms of NRT) has been found to be more effective than using a single form of NRT.13 Based on a smaller number of studies, varenicline appears to be more effective than NRT or bupropion sustained-release.13 Information on dosing regimens is available in the package inserts of individual medications or in the 2020 Surgeon General Report on Smoking Cessation.47
Pregnant Persons. Behavioral counseling interventions. Providing any psychosocial intervention to pregnant persons who smoke tobacco can increase smoking cessation. The behavioral counseling intervention type most often studied in pregnant persons who smoke was counseling. Behavioral interventions were more effective when they provided more intensive counseling, were augmented with messages and self-help materials tailored for pregnant persons, and included messages about the effects of smoking on both maternal and fetal health and strong advice to quit as soon as possible.12,13 Although smoking cessation at any point during pregnancy yields substantial health benefits for the expectant mother and infant, quitting early in pregnancy provides the greatest benefit to the fetus.12,13 Other interventions included feedback, incentives, health education, and social support, although provision of health education alone, without counseling, was not found to be effective. For additional information about behavioral counseling interventions in pregnant persons, see Table 4.12,13,15–45
ADDITIONAL RESOURCES
Primary care clinicians may find the following resources useful in talking with adults and pregnant persons about tobacco smoking cessation.
Centers for Disease Control and Prevention
Health care clinician resources for treatment of tobacco use and dependence
https://www.cdc.gov/tobaccoHCP
Tips from Former Smokers
https://www.cdc.gov/tobacco/campaign/tips/partners/health/index.html
U.S. Department of Health and Human Services
SmokeFree.Gov Health Professionals Page
https://smokefree.gov/help-others-quit/health-professionals
SmokeFreeWomen
In addition, the following resources may be useful to primary care clinicians and practices trying to implement interventions for tobacco smoking cessation.
Million Hearts tools for clinicians for tobacco cessation
https://millionhearts.hhs.gov/tools-protocols/tools/tobacco-use.html
Centers for Disease Control and Prevention state and community resources for tobacco control programs
U.S. Department of Veterans Affairs Primary Care & Tobacco Cessation Handbook
World Health Organization's toolkit for delivering brief smoking interventions in primary care
http://www.who.int/tobacco/publications/smoking_cessation/9789241506953/en/
In 2020, the Surgeon General issued a Report on Smoking Cessation.47 The report's findings were largely similar to those of the USPSTF. The Surgeon General's report issued some additional findings regarding internet-based interventions for cessation and describes some suggestive but not sufficient evidence about specific e-cigarette use behaviors and increased cessation. Overall, the Surgeon General's report found that there is inadequate evidence to conclude that e-cigarettes increase smoking cessation. More information on the Surgeon General's Report on Smoking Cessation is available at https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/#fact-sheets.
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENTS
Pharmacotherapy for Pregnant Persons. According to data from the National Vital Statistics System, in 2016, 7.2% of women who gave birth smoked cigarettes during pregnancy,6 and among 1,071 pregnant women aged 18 to 44 years, 3.6% reported using e-cigarettes.48 Smoking during pregnancy reduces fetal growth, increases the risk of preterm birth, and doubles the risk for delivering an infant with low birth weight. It also increases the relative risk for stillbirth death by 25% to 50%.1,2 Quitting smoking early in pregnancy can reduce or eliminate the adverse effects of smoking on fetal growth.47 For pregnant persons for whom behavioral counseling alone does not work, evidence to support other options to increase smoking cessation during pregnancy are limited. Few clinical trials have evaluated the effectiveness of NRT for smoking cessation in pregnant women. Although most studies were in the direction of benefit, no statistically significant increase in cessation was seen.13 There is limited evidence on harms of NRT from trials in pregnant persons. Potential adverse maternal events reported in studies of NRT include slightly increased diastolic blood pressure and skin reactions to the patch.13 Potential adverse events reported in nonpregnant adults include higher rates of low-risk cardiovascular events, such as tachycardia.13 It has been suggested that NRT may be safer than smoking during pregnancy given that cigarette smoke contains harmful substances in addition to nicotine. The USPSTF identified no studies on bupropion sustained-release or varenicline pharmacotherapy for tobacco smoking cessation during pregnancy.
In the absence of clear evidence on the balance of benefits and harms of pharmacotherapy in pregnant women, clinicians are encouraged to consider the severity of tobacco dependence in each patient and engage in shared decision-making to determine the best individual treatment course.
e-Cigarettes in Nonpregnant Adults and Pregnant Persons. No tobacco product use is risk-free, including the use of e-cigarettes. Tobacco smoking cessation can be difficult for many individuals; thus, having a variety of tools available to help persons quit smoking would potentially be helpful. Findings from small surveys and qualitative data report mixed findings on whether physicians are recommending e-cigarettes to patients to help them quit smoking.13,49–51 Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.13 Overall, results were mixed on whether smoking cessation increased with e-cigarettes; however, continued e-cigarette use after the intervention phase of trials remained high, indicating continued nicotine dependence. Trial evidence on harms of e-cigarettes used for smoking cessation is also limited. The most common adverse effects from e-cigarette use reported in trials included coughing, nausea, throat irritation, and sleep disruption.13 Generally, no significant difference in short-term serious adverse events associated with e-cigarette use was reported.13 Evidence on potential harms of e-cigarette use in general (whether for tobacco smoking cessation or not) has been reviewed in the National Academies of Sciences, Engineering, and Medicine report Public Health Consequences of E-Cigarettes.52 For example, the report found conclusive evidence that in addition to nicotine, most e-cigarette products contain and emit numerous potentially toxic substances. Additionally, an outbreak of e-cigarette, or vaping product, use–associated lung injury that occurred in the United States in late 2019 also suggests potential harms of e-cigarette use. The vast majority of cases have been associated with tetrahydrocannabinol-containing e-cigarettes.53
Given the high rates of e-cigarette use in children and adolescents currently in the United States,54 the USPSTF recognizes that an overall public health question remains on whether the potential use of e-cigarettes as a tobacco smoking cessation aid (if ever proven effective) could be balanced with the high rates of e-cigarette use in youth as a driver for increasing overall tobacco use. The USPSTF has issued a separate recommendation statement on the prevention of tobacco use, including e-cigarettes, in children and adolescents.9 The current USPSTF recommendation statement for adults evaluated the evidence on the benefits and harms of e-cigarettes to increase tobacco cessation; the USPSTF found this evidence to be insufficient. Given the proven effectiveness of behavioral counseling interventions in both nonpregnant and pregnant adults, and of pharmacotherapy in nonpregnant adults, the USPSTF recommends that clinicians focus on offering behavioral counseling and pharmacotherapy to increase smoking cessation in non-pregnant adults, and behavioral counseling to increase smoking cessation in pregnant persons.
OTHER RELATED USPSTF RECOMMENDATIONS
In 2020, the USPSTF recommended that primary care clinicians provide interventions, including education or brief counseling, to prevent the initiation of tobacco use (including e-cigarettes) in school-aged children and adolescents.9 The USPSTF found the evidence on primary care interventions for the cessation of tobacco use in youth to be insufficient.
This recommendation statement was first published in JAMA. 2021;325(3):265–279.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” “Research Needs and Gaps,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
The complete version of this statement, including supporting scientific evidence, evidence tables, grading system, members of the USPSTF at the time this recommendation was finalized, and references, is available on the USPSTF website at https:// www.uspreventive services task force.org/.
