
Am Fam Physician. 2021;104(3):222-223
Author disclosure: No relevant financial affiliations.
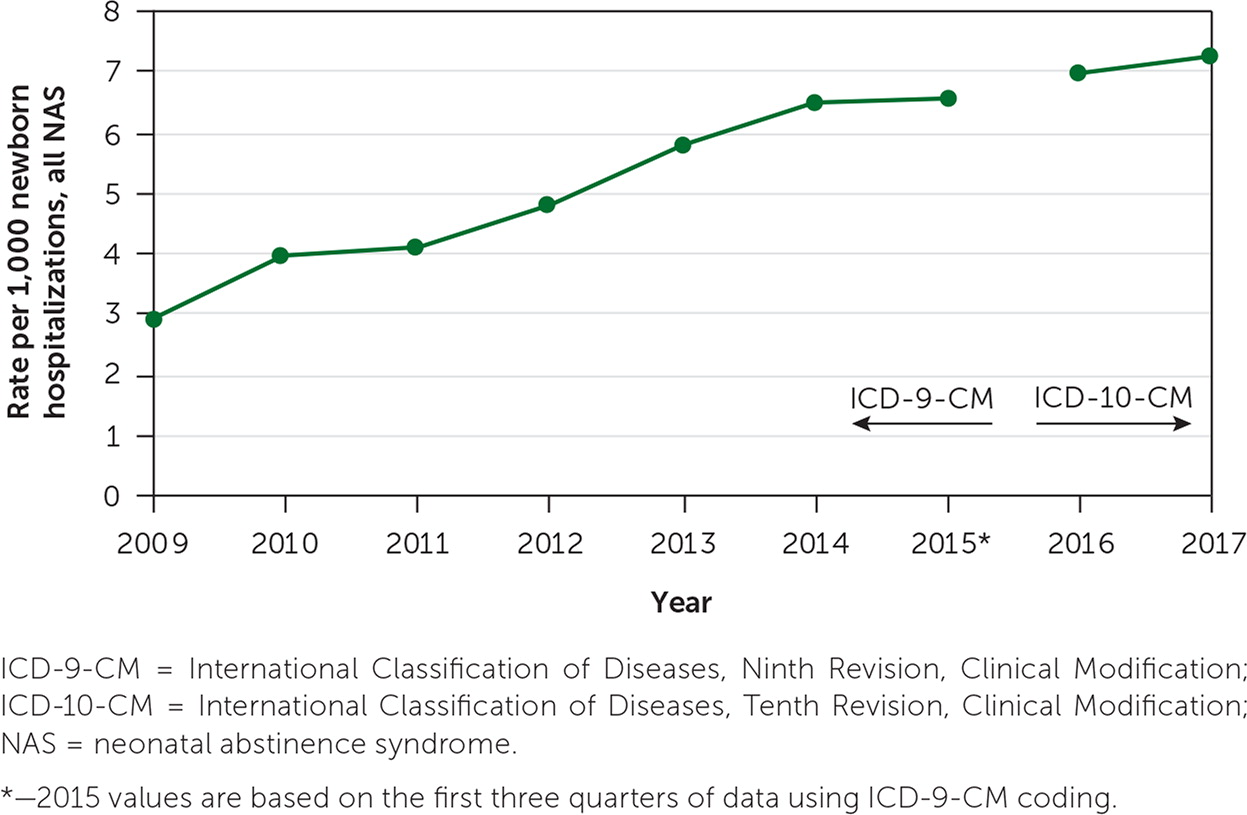
Neonatal abstinence syndrome (NAS) is a constellation of symptoms observed in newborns exposed to opioids during pregnancy.1 Between 50% and 80% of infants exposed to opioids in utero will develop NAS.2 In October 2017, the U.S. Department of Health and Human Services declared the opioid crisis in the United States a public health emergency. The incidence of NAS has steadily increased during the past decade as more infants have been born to mothers with opioid use disorder (OUD; Figure 1).3 Pregnancy and the postpartum period are critical times to advocate for women with OUD and their infants. Embracing the mother-infant dyad with love, patience, and compassion is essential for successful treatment.
The U.S. Preventive Services Task Force recommends screening for unhealthy drug use in people 18 years or older, and the American Academy of Family Physicians suggests selective screening for OUD in the same population.4,5 According to the American College of Obstetricians and Gynecologists, all pregnant people should be screened for OUD and be offered medication-assisted treatment with methadone or buprenorphine when appropriate.6 Pregnant people already receiving medication-assisted treatment should continue the medication because the risks of untreated OUD include poor fetal health, miscarriage, return to substance use, and overdose.6 Patients should be informed of the possibility of NAS and be counseled on its diagnosis, management, and consequences.
Newborns with NAS can have lower birth weights and be difficult to soothe. They may have a high-pitched cry, tremors, hypertonia, hyperactive reflexes, impaired feeding, poor weight gain, vomiting, diarrhea, mottling, and temperature instability. Diagnosis of NAS is based on a positive drug screen from meconium, urine, or umbilical cord tissue, or cord blood with a maternal history of OUD. Infants at risk should be monitored for five days using a validated NAS scale.6 The modified Finnegan NAS score (https://www.mdcalc.com/modified-finnegan-neonatal-abstinence-score-nas) uses 21 different criteria regarding central nervous system, metabolic, vasomotor, respiratory, and gastrointestinal disturbances.7,8 Three consecutive scores of 8 or greater or two consecutive scores of 12 or greater should prompt treatment for NAS.
Initial treatment includes creating a low-stimulation environment; swaddling and rocking the infant; and providing frequent, on-demand feedings to reduce infant stress and to provide adequate calories. Skin-to-skin contact increases infant comfort and promotes attachment. Breastfeeding has been associated with specific benefits for infants who were exposed to opioids in utero, including less severe NAS and reduced need for pharmacologic intervention.9
Pharmacologic treatment with opioids such as morphine or methadone is indicated for infants whose symptoms do not improve with supportive care alone within the first five days of life. A combination of morphine or methadone with phenobarbital or clonidine is indicated for infants with in utero polysubstance exposure (e.g., benzodiazepines, cannabis, cocaine, fentanyl, phencyclidine).10 Treatment with buprenorphine has been shown to result in shorter duration of treatment and length of hospital stay compared with treatment with morphine.11
A family-centered model called Eat, Sleep, Console has been developed at Yale University Hospital.12 This treatment approach focuses on maximizing the benefits of nonpharmacologic treatments first and actively involving and empowering the mother in the infant's care. An algorithm for this approach is available at https://hosppeds.aappublications.org/content/8/1/1. The implementation of this approach has significantly decreased the need for morphine treatment and the length of hospital stay, fostering the family's ability to care for the infant after discharge by being involved in the infant's care during the hospital stay.12,13
The time the infant spends in the hospital while being monitored and treated for NAS provides an opportunity for a fresh start for the mother. Mothers should be viewed as medicine for their infants; by spending time together, infants will likely need less pharmacologic treatment, hence a shorter hospital stay and decreased hospital costs. In addition, strengthening the mother-infant bond may reduce postpartum depression and improve maternal stress response.
As mothers and infants prepare to leave the hospital, a comprehensive discharge plan should address treatment for maternal substance-use disorder, a safe living environment, and parenting and community support. The infant should be referred to a dedicated family physician or pediatrician for close follow-up for the mother and infant. Short-term studies have shown that infants with NAS are at risk of developmental delay, poor academic performance, vision problems, and behavioral problems and have more frequent visits to the emergency department in the first eight years of life.14–17 All infants with NAS should receive regular primary care and be referred to early intervention services to optimize their health and developmental outcomes.
