
Am Fam Physician. 2021;104(3):314-315
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Primary HPV testing alone every five years is more sensitive for dysplasia than cytology and more specific than cotesting with HPV testing and cytology.
• Primary HPV testing every five years from 25 to 65 years of age reduces cancers and cancer deaths as much as cytology from 21 to 30 years of age and cotesting from 30 to 65 years of age while also reducing pelvic examination burden by about one-half
• Cervical cancer screening can be discontinued after 65 years of age in women at low risk due to previous negative screening.
From the AFP Editors
Cervical cancer screening programs are successful, decreasing cancer incidence dramatically since the 1950s to just less than 14,000 cases in 2020. Yet, more than 4,000 patients die annually from cervical cancer, with racial and socioeconomic disparities continuing to contribute to this number. The American Cancer Society (ACS) has released new guidelines for cervical cancer screening.
Best Screening Test
The primary goal of screening is to prevent cervical cancer by detecting treatable abnormalities and precancerous lesions. Early detection of invasive cervical cancer is a secondary goal.
In 2018, the U.S. Preventive Services Task Force (USPSTF) recommended primary human papillomavirus (HPV) testing without cytology as an option for cervical cancer screening. Both primary HPV tests are approved for patients 25 years and older. Primary HPV testing is more sensitive and has a higher long-term negative predictive value than cytology. In vaccinated patients, abnormal cytology findings are primarily caused by HPV types with low cancer risk. Cotesting with cytology and HPV testing increases false-positive results compared with primary HPV testing with minimal effect on cervical cancer detection.
Screening Recommendations
In a major shift from their 2012 guideline, the ACS recommends that patients with a cervix undergo primary HPV testing every five years, without cytology, beginning at 25 years of age and, for most patients, stopping at 65 years of age. The recommendation not to initiate screening until age 25 is based on several factors. Less than 1% of cervical cancers are diagnosed in patients younger than 25 years, and early screening does not prevent these cancers. Many abnormal findings in patients 21 to 24 years of age would regress without intervention because HPV infections in this group are commonly transient. Large observational studies suggest that screening younger patients offers little benefit in reducing cervical cancer, and treatment can increase risks of preterm birth.
Primary HPV testing is not yet available in many locations. Until it is an option, screening can be performed through cotesting every five years or cytology alone every three years.
Although vaccination reduces risks of positive HPV test results and precancerous lesions, evidence is insufficient to modify screening recommendations for vaccinated patients.
When to Stop Cervical Cancer Screening
Although one in five cervical cancer deaths occurs in patients older than 65 years, most were diagnosed at a younger age or had insufficient screening. Testing beyond this age in previously screened patients adds little benefit with a cost of more colposcopies. Cervical cancer screening is no longer required in low-risk patients older than 65 years who have not been diagnosed with grade 2 or greater cervical intraepithelial neoplasia within the past 25 years and who have had negative screening results for the past 10 years using any recommended interval.
Benefits and Risks
Based on models, there will be similar mortality and fewer cancers with five-year primary HPV screening compared with screening using previous recommendations as shown in Table 1. The new recommendations reduce screening by nearly one-half for each patient, but slightly increase colposcopy rates.
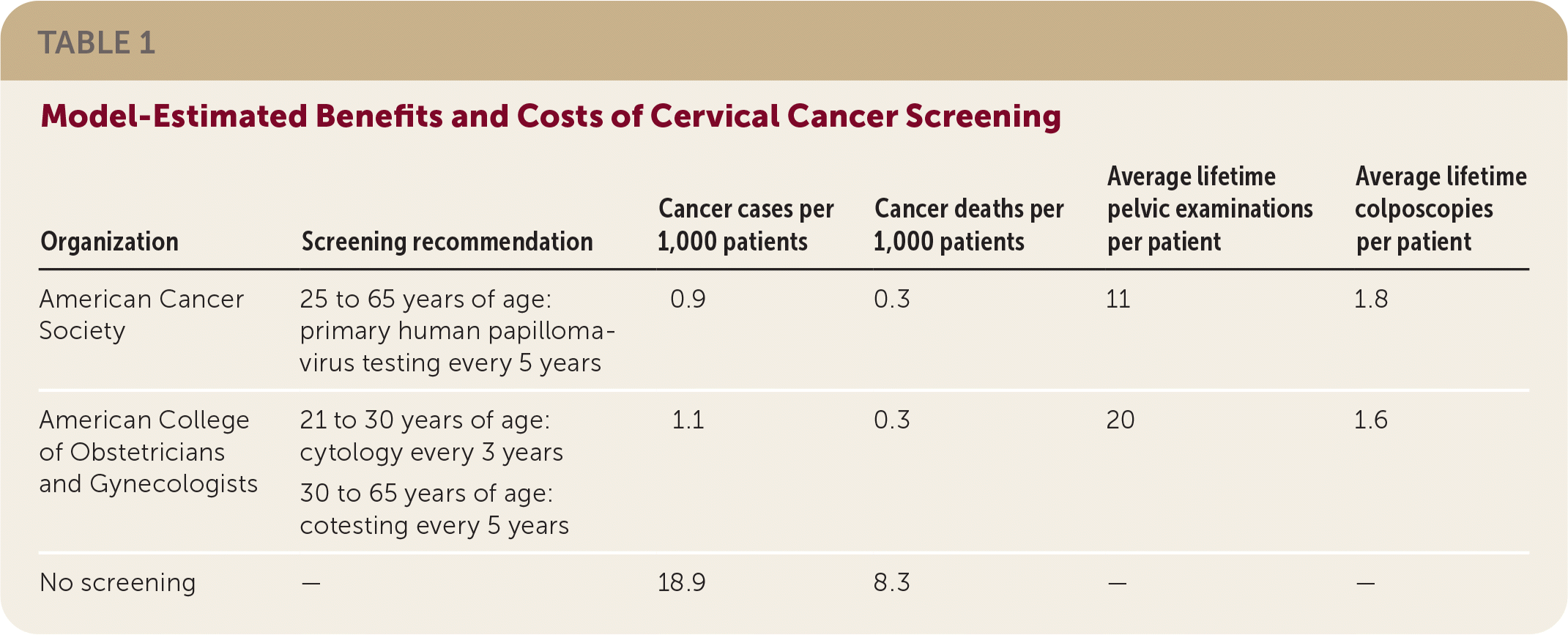
| Organization | Screening recommendation | Cancer cases per 1,000 patients | Cancer deaths per 1,000 patients | Average lifetime pelvic examinations per patient | Average lifetime colposcopies per patient |
|---|---|---|---|---|---|
| American Cancer Society | 25 to 65 years of age: primary human papillomavirus testing every 5 years | 0.9 | 0.3 | 11 | 1.8 |
| American College of Obstetricians and Gynecologists | 21 to 30 years of age: cytology every 3 years 30 to 65 years of age: cotesting every 5 years | 1.1 | 0.3 | 20 | 1.6 |
| No screening | — | 18.9 | 8.3 | — | — |
Some concerns have been raised with the longer screening interval. The burden of cervical cancer is higher in Black and Hispanic patients, who are underrepresented in trials. Longer screening intervals can reduce adherence, especially in patients from racial and ethnic minority groups or patients with limited access to health care.
Editor's Note: Dr. Auguste served as student representative to AFP.
The ACS cervical cancer screening recommendations are simpler than previous recommendations and represent a shift away from cytology (e.g., Papanicolaou smears). Benefits include reducing preterm delivery by eliminating unnecessary treatments in young patients and the number of lifetime screening pelvic examinations.
In contrast, the American College of Obstetricians and Gynecologists (ACOG) continues to recommend screening starting at 21 years of age, and expresses concern that delaying screening until age 25 could further reduce screening rates in patients younger than 30 years and worsen health inequities.1
An estimated 130 U.S. patients die from cervical cancer before they reach age 30 each year, making it the second most lethal cancer for patients in this age group.2 Although nearly 50% of adolescents have been vaccinated for HPV, with the highest rates in Black and Hispanic adolescents, the effects of vaccination on cancer incidence and health disparities are not clear.2 ACOG does recognize primary HPV screening as an option in patients 30 years and older. The conflicting recommendations demonstrate the limited evidence base and the variety of reasonable approaches to cancer prevention.—Michael J. Arnold, MD, Contributing Editor
References
1. American College of Obstetricians and Gynecologists. Updated cervical cancer screening guidelines. Practice advisory. April 2021. Accessed May 31, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines
2. Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229–232.
Guideline source: American Cancer Society
Evidence rating system used? No
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: CA Cancer J Clin. September 2020;70(5):321–346.
