Am Fam Physician. 2021;104(5):517-518
Author disclosure: No relevant financial affiliations.
The Aptima Mycoplasma genitalium assay is a nucleic acid amplification test (NAAT) for the detection of ribosomal RNA from M. genitalium. It is used to detect this common bacterial cause of sexually transmitted infections (STIs) in men and women using first-void urine or vaginal, endocervical, penile-meatal, or urethral swabs.1 The assay was approved by the U.S. Food and Drug Administration in 2019 and is the only NAAT approved for M. genitalium infection. M. genitalium is estimated to cause 15% to 25% of nongonococcal urethritis and is a common cause of recurrent urethritis in the United States.2
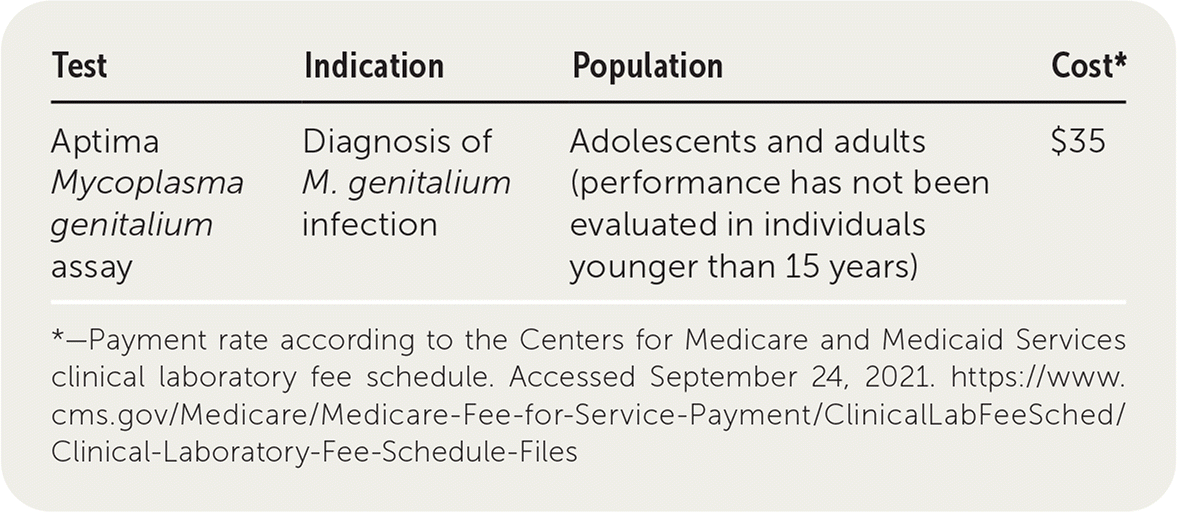
| Test | Indication | Population | Cost* |
|---|---|---|---|
| Aptima Mycoplasma genitalium assay | Diagnosis of M. genitalium infection | Adolescents and adults (performance has not been evaluated in individuals younger than 15 years) | $35 |
Accuracy
A 2019 prospective study conducted at 21 sites in the United States compared the Aptima M. genitalium assay with a composite reference of three transcription-mediated NAATs targeting unique regions of M. genitalium 16S or 23S ribosomal RNA.3 Seven different specimen types were analyzed from 1,789 females and 1,604 males (self-collected first-catch urine, self-collected vaginal swab, clinician-collected vaginal swab, and clinician-collected endocervical swab for females; self-collected penile-meatal swab, clinician-collected urethral swab, and self-collected first-catch urine for males).
Overall prevalence of M. genitalium infection was 12.0% and 8.8% among symptomatic and asymptomatic participants, respectively. 3 The sensitivity of the assay was 90% or greater for clinician- and patient-collected vaginal and male urethral swab specimens and male urine specimens, 88.4% for penile-meatal specimens, 81.5% for endocervical specimens, and 77.8% for female urine specimens. Specificity was 97.8% or higher for all specimen types. Sensitivity and specificity were similar in asymptomatic and symptomatic participants.3 The Aptima M. genitalium assay package insert notes that for female patients, a vaginal swab is the preferred specimen type because of higher sensitivity; however, alternative specimens may be used when a vaginal swab is unavailable.4
In a separate study, a composite reference standard comprising three other transcription-mediated amplification tests was used to evaluate the performance of the Aptima M. genitalium assay on vaginal swab, female urine, and male urine specimens from 1,400 adults at three sites in the United States.5 The sensitivity, specificity, and overall agreement of the assay were 100%, 99.9%, and 99.9%, respectively.5
A 2017 prospective study including 1,431 clinical specimens from 1,235 symptomatic and asymptomatic patients evaluated the performance of the assay compared with in-house real-time polymerase chain reaction testing.6 The sensitivity of the assay was significantly higher than the polymerase chain reaction test (100% vs. 59.7%), and its specificity was 99.1%.6
Benefit
The Aptima M. genitalium assay offers accurate detection of this increasingly common STI. Samples can be easily collected in a clinical setting, and patients can also be taught to self-collect samples. Prior detection of Mycoplasma using traditional microbiological methods was difficult and would take up to six months to culture.7 The 2021 Centers for Disease Control and Prevention treatment guidelines for STIs recommend using NAAT testing in men with recurrent or persistent nongonococcal urethritis and in women with recurrent cervicitis and considering testing in women with pelvic inflammatory disease.8 Screening asymptomatic men or women for M. genitalium infection and extragenital testing are not recommended.8
Testing for M. genitalium resistance to macrolide or quinolone is currently under investigation and not commercially available in the United States. Although more rapid detection may facilitate timely antibiotic treatment, no studies have demonstrated improved patient outcomes from the use of this test.
Harms
The lower sensitivity of female urine and endocervical specimens may lead to false-negative test results and delayed treatment. Therefore, repeat testing of a vaginal sample may be indicated, leading to increased costs.
Cost
The 2021 Centers for Medicare and Medicaid Services Clinical Laboratory Fee Schedule reimburses $35 for the detection of M. genitalium by DNA or RNA probe.9 Additional costs may be decreased if testing facilitates diagnosis and appropriate antimicrobial treatment, whereas additional costs may be increased if the test is used indiscriminately in low-risk populations. No studies have evaluated the cost-effectiveness of routinely testing asymptomatic men or women with risk factors for M. genitalium infection.
Bottom Line
The Aptima M. genitalium assay is highly sensitive and specific. The Centers for Disease Control and Prevention recommends the use of NAAT testing for M. genitalium infection in men with recurrent nongonococcal urethritis, in women with recurrent cervicitis, and possibly in women with pelvic inflammatory disease. For laboratories that already use the Aptima Panther testing platform, testing for M. genitalium infection, chlamydia, and gonorrhea can be provided using the same sample when appropriate. More studies are needed to determine cost-effectiveness of the Aptima M. genitalium assay and to confirm that the test improves patient outcomes.