
Am Fam Physician. 2022;105(1):10-12
Related AFP Community Blog: Newer Glucose-Lowering Drugs Also Treat Obesity and Heart Failure
Author disclosure: No relevant financial relationships.
Treatment options for patients with type 2 diabetes mellitus have increased with the addition of three new drug classes: sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl-peptidase-4 (DPP-4) inhibitors. Large clinical trials have investigated cardiovascular outcomes with all three classes in patients who have diabetes, both those with established cardiovascular disease (CVD) and those at high risk of cardiovascular events (age older than 60 years and at least one additional cardiovascular risk factor).1–4 The primary outcome in these trials was a composite of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular mortality (major adverse cardiac events [MACE]). We should consider these patient-oriented outcomes when choosing medications for our patients with type 2 diabetes. Applying these data to patients with diabetes who are at lower cardiovascular risk can be challenging and should incorporate patient-centered shared decision-making.
Diabetes guidelines from major organizations support adding SGLT-2 inhibitors and/or GLP-1 agonists to metformin therapy for patients with type 2 diabetes and established CVD,5–10 and most guidelines support their use in patients at high risk of cardiovascular events.6–8 In patients with established CVD, reduced ejection fraction heart failure, or stage 2 or 3 chronic kidney disease, GLP-1 agonists and SGLT-2 inhibitors were shown to be superior to placebo (Table 1),1,2,4 and DPP-4 inhibitors were noninferior to placebo3 for cardiovascular and renal outcomes. When patients at high risk, but without established CVD, were analyzed independently, the benefits were not statistically significant.1,2,4
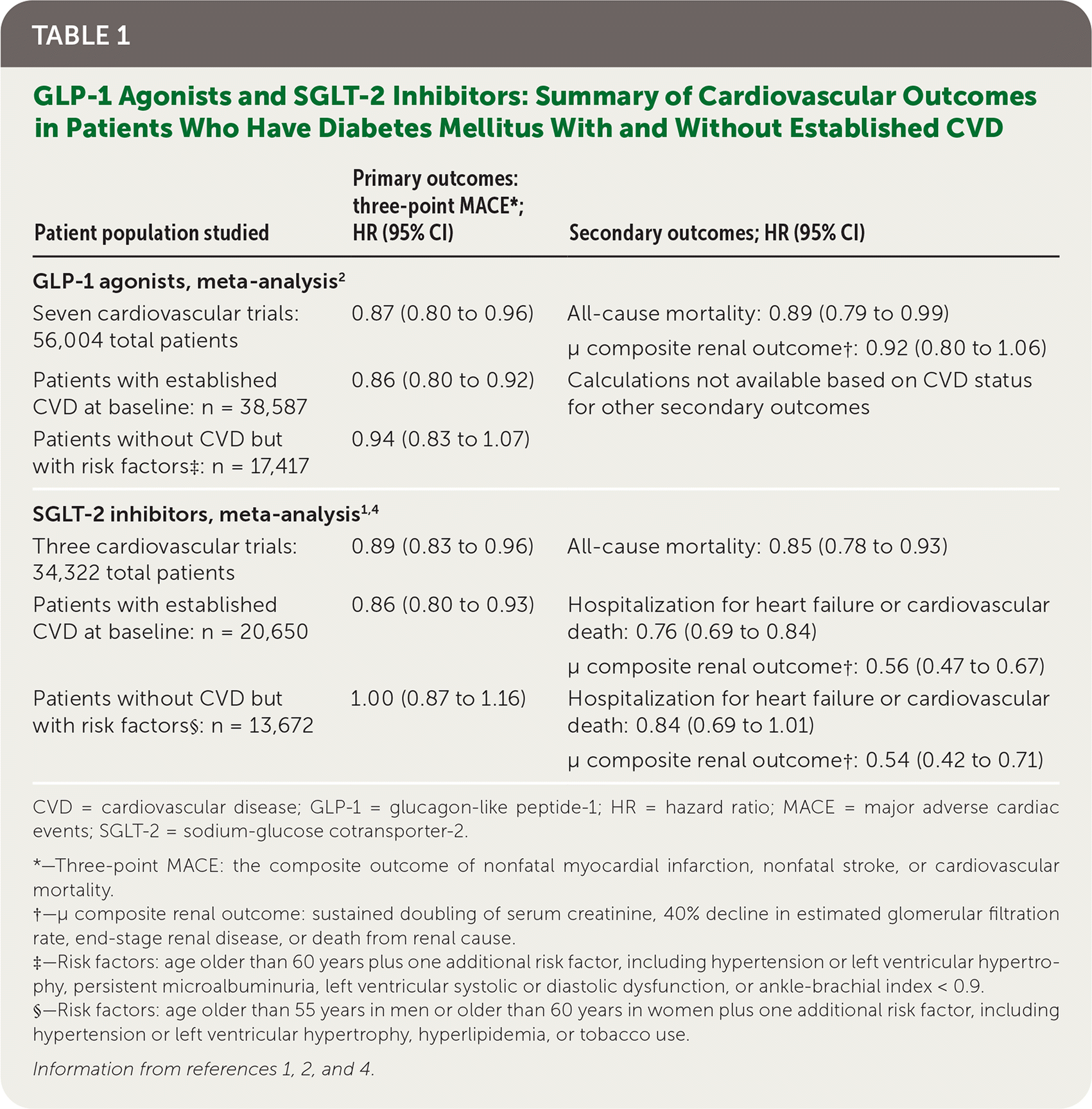
| Patient population studied | Primary outcomes: three-point MACE*; HR (95% CI) | Secondary outcomes; HR (95% CI) |
|---|---|---|
| GLP-1 agonists, meta-analysis2 | ||
| Seven cardiovascular trials: 56,004 total patients | 0.87 (0.80 to 0.96) | All-cause mortality: 0.89 (0.79 to 0.99) μ composite renal outcome†: 0.92 (0.80 to 1.06) |
| Patients with established CVD at baseline: n = 38,587 | 0.86 (0.80 to 0.92) | Calculations not available based on CVD status for other secondary outcomes |
| Patients without CVD but with risk factors‡: n = 17,417 | 0.94 (0.83 to 1.07) | |
| SGLT-2 inhibitors, meta-analysis1,4 | ||
| Three cardiovascular trials: 34,322 total patients | 0.89 (0.83 to 0.96) | All-cause mortality: 0.85 (0.78 to 0.93) |
| Patients with established CVD at baseline: n = 20,650 | 0.86 (0.80 to 0.93) | Hospitalization for heart failure or cardiovascular death: 0.76 (0.69 to 0.84) μ composite renal outcome†: 0.56 (0.47 to 0.67) |
| Patients without CVD but with risk factors§: n = 13,672 | 1.00 (0.87 to 1.16) | Hospitalization for heart failure or cardiovascular death: 0.84 (0.69 to 1.01) μ composite renal outcome†: 0.54 (0.42 to 0.71) |
A 2021 BMJ clinical practice guideline provided recommendations for the use of SGLT-2 inhibitors and GLP-1 agonists based on a network meta-analysis of 764 randomized trials with more than 400,000 participants.11,12 The BMJ guideline's recommendations, from an international panel of clinicians, methodologists, and patient partners, focused on eight key outcomes: five regarding benefit (all-cause mortality, myocardial infarction, stroke, end-stage renal disease, and hospital admission for heart failure) and three regarding adverse effects of the medications (diabetic ketoacidosis, genital infections, and severe gastrointestinal events).11 Absolute risk reduction models for CVD and kidney disease in patients with very low to very high cardiovascular risk were tabulated. Patient perspectives on harms and burdens were also considered. A strong recommendation was given only when a clear benefit was determined, whereas a weak recommendation represented a finer balance among benefits, harms, and burdens of treatment options.11
The BMJ guideline made a weak recommendation for the use of SGLT-2 inhibitors and GLP-1 agonists for the primary prevention of MACE in patients at high risk but without established CVD; a strong recommendation was made for the use of SGLT-2 inhibitors in patients with established CVD or kidney disease. Benefits varied widely based on cardiovascular risk. For example, absolute calculated differences in all-cause mortality ranged from five fewer deaths per 1,000 patients over five years in the lowest cardiovascular risk group to 48 fewer deaths in the highest cardiovascular risk group.11,12
The BMJ guideline differs somewhat from other organizations. The American Diabetes Association gives a grade A recommendation for prescribing an SGLT-2 inhibitor or a GLP-1 agonist for patients with established CVD, multiple cardiovascular risk factors, or diabetic kidney disease.6 The American College of Cardiology and American Heart Association consider both classes reasonable for patients who have diabetes with established CVD, but they do not comment on the use of these medications for primary prevention in patients at high risk of MACE.9
Treatment decisions in people with type 2 diabetes should incorporate careful evaluation of risk factors and a patient-centered shared decision-making approach. Current data strongly support a reduction in MACE and all-cause mortality with SGLT-2 inhibitors and GLP-1 agonists in patients who have diabetes with established CVD or kidney disease.2–4 These patients should be offered one of these medications in the absence of contraindications, regardless of glucose control. Because there is no significant reduction in cardiovascular outcomes in patients who have diabetes without established CVD, patient-centered shared decision-making about adding an SGLT-2 inhibitor or a GLP-1 agonist for cardiovascular benefit is important and should emphasize the lack of firm data. Patients who have a lower cardiovascular risk should consider SGLT-2 inhibitors or GLP-1 agonists based on other established benefits such as weight control (moderate net weight loss for GLP-1 agonists and small weight loss for SGLT-2 inhibitors) against risks of genital infections (SGLT-2 inhibitors) or gastrointestinal disturbances (GLP-1 agonists).
The benefits and risks of other glucose-lowering options such as DPP-4 inhibitors, insulin, and sulfonylureas should also be part of these conversations. Although DPP-4 inhibitors have a strong safety profile, they did not improve cardiovascular outcomes in large clinical trials of patients who have diabetes, mostly those with established CVD, and they are expensive.3
Family physicians must be equipped to guide patients with diabetes to appropriate medication management, which is not an easy task given the abundance of data. Relying solely on guideline recommendations without understanding the limitations of the primary literature may result in overprescribing.
