
Am Fam Physician. 2022;105(1):79-81
Author disclosure: No relevant financial relationships.
PrecivityAD is a blood test marketed to aid in the diagnosis of Alzheimer disease in patients 60 years or older with cognitive impairment. It is currently available in 47 states, the District of Columbia, and Puerto Rico. PrecivityAD is not approved by the U.S. Food and Drug Administration (FDA) but received breakthrough device designation from the FDA in 2019.

| Test | Indication | Population | Cost* |
|---|---|---|---|
| PrecivityAD | Evaluation for Alzheimer disease | Patients 60 years and older with mild cognitive impairment | $1,250 |
Using mass spectrometry, the PrecivityAD test quantifies the concentration ratio of two amyloid-beta (Abeta) peptides, Abeta 42 and 20, and detects apolipoprotein E (apoE) genotype. These markers help determine the diagnostic probability of Alzheimer disease.1,2 These values, along with the patient's age, are used in a proprietary algorithm to provide an Amyloid Probability Score (APS). The APS is stratified as low (0 to 35) or high (58 to 100); a score of 36 to 57 is considered intermediate and requires further evaluation. A high APS indicates a higher likelihood that the patient will have amyloid plaques on amyloid positron emission tomography (PET).3
Accuracy
The APS produced by PrecivityAD testing predicts the likelihood of an amyloid-positive PET result, with its accuracy having been evaluated in low-quality studies. Amyloid PET is highly sensitive (91%) and specific (92%) for distinguishing autopsy-confirmed Alzheimer disease from non–Alzheimer disease dementia.4 In those with mild cognitive impairment, amyloid PET imaging may also help with prognostication because positive imaging findings suggest a higher chance of conversion to Alzheimer disease.5
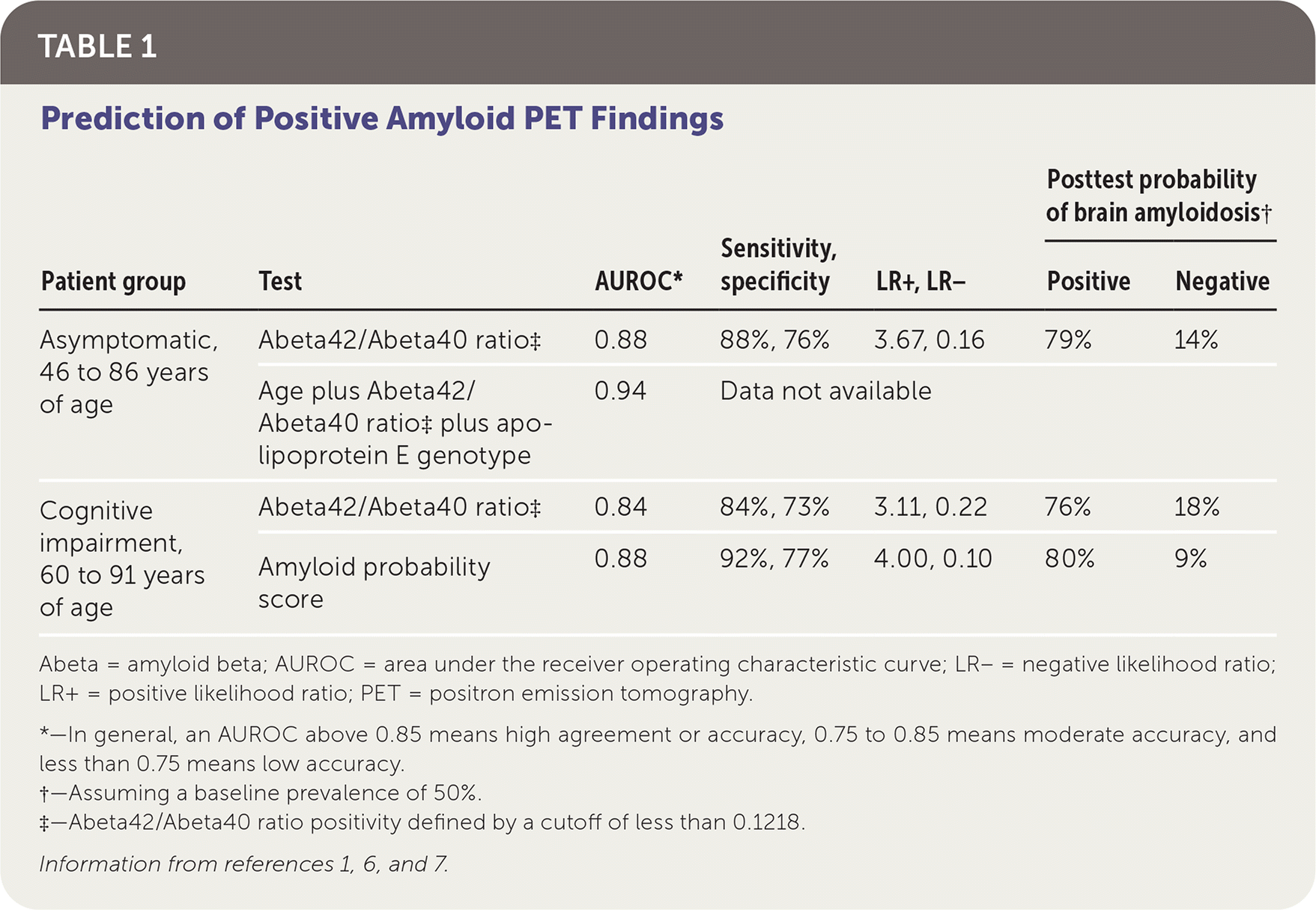
Table 1 shows the results of two studies evaluating the diagnostic accuracy of the APS and its components.1,6,7 In a prospective cohort study, 210 samples from 158 patients with normal cognition who underwent amyloid PET within the previous 18 months were analyzed for the Abeta42/Abeta20 ratio and apoE genotype. Using a ratio cutoff of less than 0.1218 to define positivity, a positive result was predictive of positive amyloid PET results. Combining the plasma Abeta42/Abeta40 ratio, patient age, and apoE genotype improved concordance with amyloid PET findings.1 A subgroup analysis of 74 patients with negative amyloid PET findings initially showed that those with a positive plasma Abeta42/Abeta20 ratio had a 15-fold higher risk of developing brain amyloidosis seen on amyloid PET compared with those who had a negative ratio (mean duration after baseline serologic testing and last amyloid PET scan was 3.9 ± 1.4 years, with a range of 1.9 years to 9.0 years).1
| Patient group | Test | AUROC* | Sensitivity, specificity | LR+, LR− | Posttest probability of brain amyloidosis† | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Asymptomatic, 46 to 86 years of age | Abeta42/Abeta40 ratio‡ | 0.88 | 88%, 76% | 3.67, 0.16 | 79% | 14% |
| Age plus Abeta42/Abeta40 ratio‡ plus apolipoprotein E genotype | 0.94 | Data not available | ||||
| Cognitive impairment, 60 to 91 years of age | Abeta42/Abeta40 ratio‡ | 0.84 | 84%, 73% | 3.11, 0.22 | 76% | 18% |
| Amyloid probability score | 0.88 | 92%, 77% | 4.00, 0.10 | 80% | 9% | |
In an unpublished study of PrecivityAD that has not undergone peer review, researchers analyzed blood samples and amyloid PET results from 686 patients 60 to 91 years of age (mean age of 73 years) with cognitive impairment, for whom there was clinical suspicion for Alzheimer disease.7 In this cohort, the Abeta42/Abeta40 ratio alone accurately predicted findings on amyloid PET, and accuracy increased when using the calculated APS. Based on these data, cutoff values for the APS were identified (lower cutoff of 0 to 35 and upper cutoff of 58 to 100), which allowed for exclusion of those with scores in the intermediate range (36 to 57) and improved accuracy of the test.7 An estimated 10% to 15% of patients will have an APS within the intermediate range and need further evaluation.8
Because the clearance of amyloid and apoE peptides can be reduced in certain conditions, including chronic kidney disease and obesity, the accuracy of this testing may be reduced in certain patient populations.7
Benefit
Based on the accuracy of PrecivityAD in identifying patients with a high likelihood of brain amyloidosis, this test may reduce the need for the more costly and invasive cerebrospinal fluid testing that is currently used to identify Alzheimer disease biomarkers. It also may reduce the number of PET scans performed, which would decrease radiation exposure and cost.
The PrecivityAD test could reduce the number of confirmatory tests required to identify clinical research participants with brain amyloidosis. For example, rates of negative amyloid PET findings are approximately 70% in Alzheimer disease prevention trials.9 Prescreening participants using the plasma Abeta42/Abeta40 ratio is estimated to reduce the number of required amyloid PET scans by 62%.1
Harms
Although results of the PrecivityAD test appear to accurately correlate with amyloid PET findings in older adults with mild cognitive impairment, its role and accuracy in diagnosing or predicting Alzheimer disease are unknown. The disease course and prognosis of those with cognitive impairment and positive amyloid PET findings are uncertain, potentially resulting in significant patient and caregiver anxiety. High-quality evidence supporting interventions to prevent or delay the onset of Alzheimer disease in those with mild cognitive impairment is lacking, and positive PrecivityAD test results may result in unnecessary and potentially ineffective interventions. Additionally, the implications of an APS score between 36 and 57 are unclear, and a lack of clear guidance for patients with scores in this range could lead to increased anxiety.
Cost
Bottom Line
The PrecivityAD test is a widely available blood test for older persons with mild cognitive impairment and is marketed to aid in the diagnosis of Alzheimer disease. The APS produced by the test is accurate in predicting the likelihood of positive PET findings, which may reduce the need for amyloid PET imaging. Further research is needed to determine whether use of the PrecivityAD test improves detection and clinical outcomes in patients with Alzheimer disease.
