AAFP Welcomes Proposed Graphic Cigarette Warning Labels
Long-delayed Cautions Also Belong on E-products, Academy Says
October 08, 2019 05:36 pm News Staff – A proposed FDA rule that would add stark new warning labels to cigarette packs next year deserves kudos, the Academy said in a recent letter -- but it also should spur regulators to step up safeguards on all nicotine delivery systems.
The updated labels would "promote greater public understanding of the negative health consequences of cigarette smoking," the AAFP said in its Oct. 1 letter(2 page PDF) to FDA Acting Commissioner Norman Sharpless, M.D., and HHS Deputy Secretary Eric Hargan, J.D. The correspondence, signed by Board Chair John Cullen, M.D., of Valdez, Alaska, cited a 2016 study that found visuals such as those in the proposed rule are more influential in motivating smokers to quit than text warnings and can be effective at discouraging tobacco use by youth.
The proposed rule, published in the Aug. 16 Federal Register, is the latest attempt to implement a provision of the Family Smoking Prevention and Tobacco Control Act of 2009 requiring that cigarette packaging and advertising graphically illustrate the dangers of smoking. The government withdrew an initial set of labels in 2013 following successful legal challenges by a coalition of tobacco companies that claimed the visceral images on those labels violated their First Amendment rights.
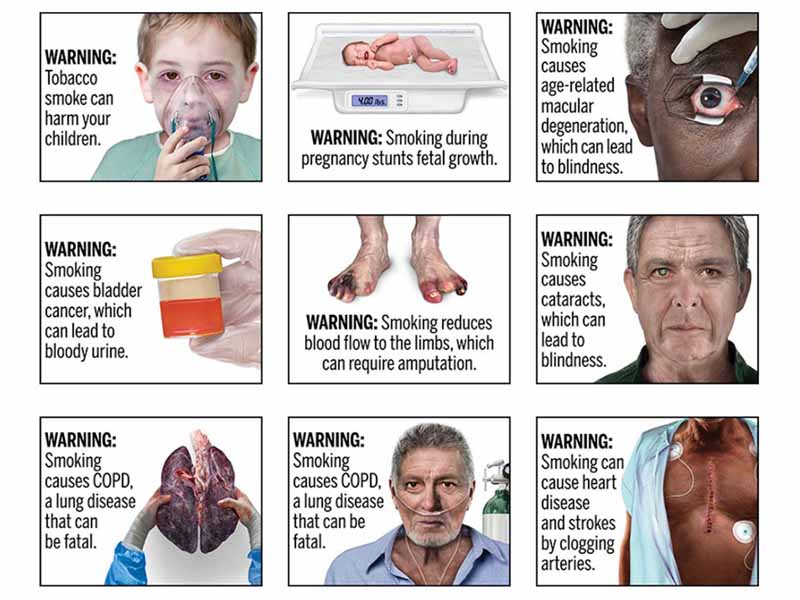
The FDA is proposing to require photo-realistic color images on cigarette packages to illustrate lesser-known health risks of smoking.
In the decade since the law was passed, e-cigarette products -- most prominently, JUUL -- have overtaken cigarettes as the dominant product by which U.S. minors are becoming addicted to nicotine. The result, the Academy recently told the White House, has been an epidemic -- one that demands special attention.
"The FDA should require child-resistant packaging for liquid nicotine and all other novel tobacco products," the Academy said in its Oct. 1 letter, reiterating previous AAFP guidance. "Given that nicotine is an addictive drug, child-resistant packaging and graphical warnings are immediate and common-sense steps that manufacturers should be required to take to prevent infants and children from inadvertently consuming or being exposed to liquid nicotine."
The letter also repeated AAFP calls for robust federal research to assess the safety, quality and efficacy of e-cigarettes as smoking-cessation devices, and again said that regulators should immediately halt the marketing and advertising of nicotine delivery devices, especially to children and youth.
The proposed rule is slated to become final next March, with the warnings showing up on cigarette packs and in advertisements 15 months after that -- if the policy goes uncontested in court. The New York Times reported in August that R.J. Reynolds Tobacco was reviewing the proposed rule.
For its part, the Academy's letter urged the FDA to leverage its full jurisdiction "to regulate the manufacture, sale, labeling, distribution and marketing of tobacco products and nicotine delivery devices, including e-cigarettes."