
Finger-stick blood glucose measurements provide a snapshot in time. Continuous glucose monitoring gives patients with diabetes a bigger picture.
Fam Pract Manag. 2021;28(2):7-14
Author disclosures: no relevant financial affiliations disclosed.

Continuous glucose monitoring (CGM) has emerged in the past decade as an important tool to gain insight into patients' glycemic patterns and trends. While self-monitoring of blood glucose with periodic finger sticks can capture glucose as a snapshot in time, CGM has the advantage of providing continuous measurements at 5- to 15-minute intervals. This gives patients and their doctors much more data to work with as they tailor diets, lifestyle, and medications to manage diabetes.
The benefits of CGM have been established in numerous studies of patients with either Type 1 or Type 2 diabetes. They include improvement in A1C, reduction of hypoglycemic events, reduction of glycemic variability, and improvement in diabetes-related distress.1–4 This article outlines how to set up a CGM program in your practice and get paid for it.
KEY POINTS
Continuous glucose monitoring (CGM) gives patients and primary care clinicians more data about blood glucose levels throughout the day than traditional finger-stick measurements.
CGM helps patients and clinicians identify blood glucose trends, making it easier to tailor diets and manage medications, and providing motivation to increase physical activity.
There are several CGM devices on the market, all with startup costs that are attainable for most practices. A series of CPT codes provide reimbursements to recoup those costs.
PERSONAL VS. PROFESSIONAL CGM
CGM devices are made of three parts: a sensor to detect interstitial glucose that is inserted subcutaneously by the patient or health care professional, a receiver or reader that displays the current glucose level, and a transmitter that sends data from the sensor to the receiver. CGM devices fall into two categories: personal use and professional use. Patients may purchase their own personal use CGM devices if they have Type 1 diabetes, uncontrolled Type 2 diabetes on intensive insulin regimens, or erratic hypoglycemia episodes (Editor's note 9/18/2023: Medicare no longer requires an intensive insulin regimen for CGM services coverage. The Medicare criteria is now that the patient be "insulin-treated.") The devices help patients learn to respond to glycemic fluctuations and increase confidence in blood sugar management. They also allow patients to share their data remotely with family members, caregivers, or clinicians.
An in-depth review of personal CGM patient selection and device comparison has been published elsewhere.5 No long-term studies have been performed to determine whether personal use CGM improves clinical outcomes in patients with Type 2 diabetes; however, evidence suggests both personal and professional CGM improve glycemic control by increasing time in range.6,7
Professional use CGM systems, which will be the focus of most of this article, are clinic-owned devices that can be placed on patients for intermittent or short-term use as a diagnostic and clinical decision-making tool. Professional CGM can be useful when patients fear hypoglycemia or fear intensifying their medication regimen. It can give patients and their clinicians important data to help them recognize patterns that lead to hypoglycemia or hyperglycemia and help them calibrate insulin doses. Professional CGM has been shown to assist patients in lowering their A1C, as well as improving their physical activity and making other positive behavior changes.8,9 The 2021 American Diabetes Association standards of care state that using CGM can help identify and correct unhealthy blood sugar levels and improve A1C levels in people with diabetes on noninsulin regimens and basal insulin regimens.10
Professional CGM can be beneficial for patients who aren't interested in personal CGM (or do not qualify for it), or it may serve as a trial run for patients considering a personal CGM device.
PRODUCT OPTIONS
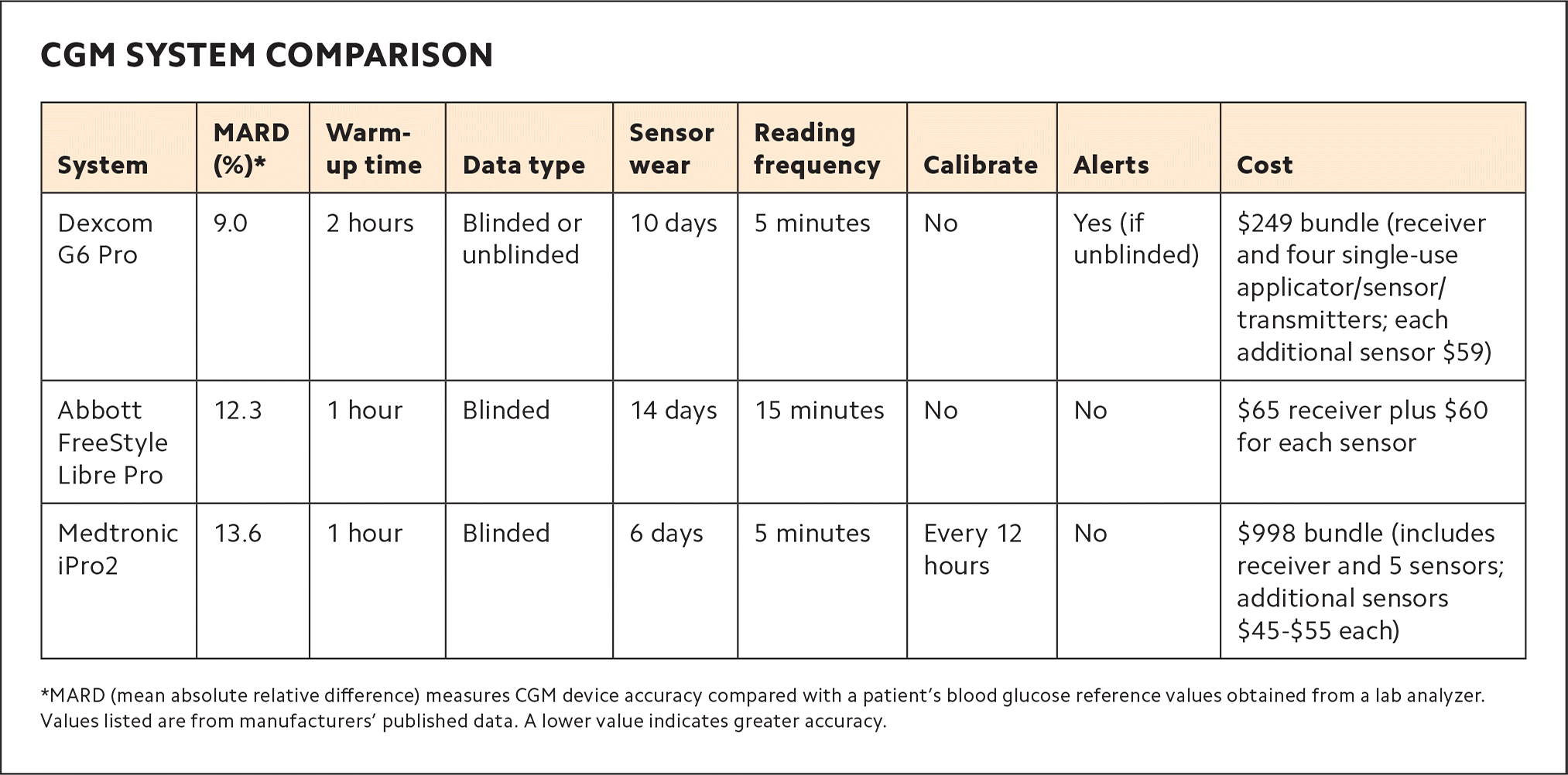
There are currently three devices available for professional CGM: the Abbott FreeStyle Libre Pro, the Dexcom G6 Pro, and the Medtronic iPro2. The three systems vary in terms of how long a patient can wear the sensor before it must be replaced (“sensor wear”), the ability to see the actual glucose number in real-time (“unblinded” mode), the ability to set alerts for high or low blood sugar, and cost.11 (See “CGM system comparison.”)
The Dexcom G6 Pro is the only one of the three devices that allows patients to view their data and receive alerts in real time if they have a compatible smart device, which acts as a receiver. The other two models are “blinded,” which means patients cannot see their data until it is downloaded by the dedicated receiver, which stays at the clinic.
The three devices also have different purchasing options, such as bundled starter kits or à la carte sensor and receiver buying. The Dexcom G6 Pro comes in a $249 starter bundle with a receiver and four single-use applicator/sensor/transmitters. Additional sensors cost $59 each. The Abbott FreeStyle Libre Pro has the lowest initial cost, offering a $65 reader device that is used to scan-start multiple patients. Each patient needs just one sensor, and they currently sell for $60. But because the FreeStyle Libre Pro's data is blinded, patients are not able to make behavior modifications based on glucose readings on their own. They must wait until the data has been downloaded and analyzed by their clinician.
Medtronic's iPro2 can be purchased in a startup bundle (which includes a receiver and five sensors) for $998. Additional sensors range from $45–55 each, based on the volume ordered. Medtronic also offers a monthly subscription program that features unlimited sensors and insurance verification services. But the Medtronic device's glucose numbers are also blinded to the patient, and it requires three finger-stick glucose numbers to be entered daily in addition to the data gathered by the sensor.
The three devices also have different data analysis software. We recommend reviewing your organization's policies about cloud-based software and internet firewalls with an IT specialist before selecting a device, to ensure you will be able to download CGM data without purchasing new technology. Representatives of device manufacturers are able to provide information about technical support, customer service, and implementation. Meeting with all three device vendors before purchasing one may be helpful.
| System | MARD (%)* | Warm-up time | Data type | Sensor wear | Reading frequency | Calibrate | Alerts | Cost |
|---|---|---|---|---|---|---|---|---|
| Dexcom G6 Pro | 9.0 | 2 hours | Blinded or unblinded | 10 days | 5 minutes | No | Yes (if unblinded) | $249 bundle (receiver and four single-use applicator/sensor/transmitters; each additional sensor $59) |
| Abbott FreeStyle Libre Pro | 12.3 | 1 hour | Blinded | 14 days | 15 minutes | No | No | $65 receiver plus $60 for each sensor |
| Medtronic iPro2 | 13.6 | 1 hour | Blinded | 6 days | 5 minutes | Every 12 hours | No | $998 bundle (includes receiver and 5 sensors; additional sensors $45–$55 each) |
SETTING UP CGM IN YOUR PRACTICE
Here's a step-by-step process for adding any of these devices to your practice.
Get administrative buy-in. Administrative support is one of the key elements for successfully implementing a clinic-owned CGM program. Administrators will need to understand the challenges of treating diabetes and the benefits of CGM. Mapping out the billing and reimbursement process, staffing needs, and projected use before purchasing CGM systems can make administrators more likely to buy in.
Designate a staff champion. Another key to a successful CGM program is identifying a staff champion within the clinic. This role can be filled by a medical assistant, licensed vocational nurse, dietitian, or diabetes educator. The main qualifications are enthusiasm for new projects and good patient education skills. The staff champion will be trained to select patients for the CGM program (see more on that below), use and maintain devices, insert sensors and provide instructions to patients, download and disseminate the results, and bill and code for services. Device manufacturers can provide training videos and educational materials for staff. Training a backup staff member may be helpful for when the champion is unavailable.
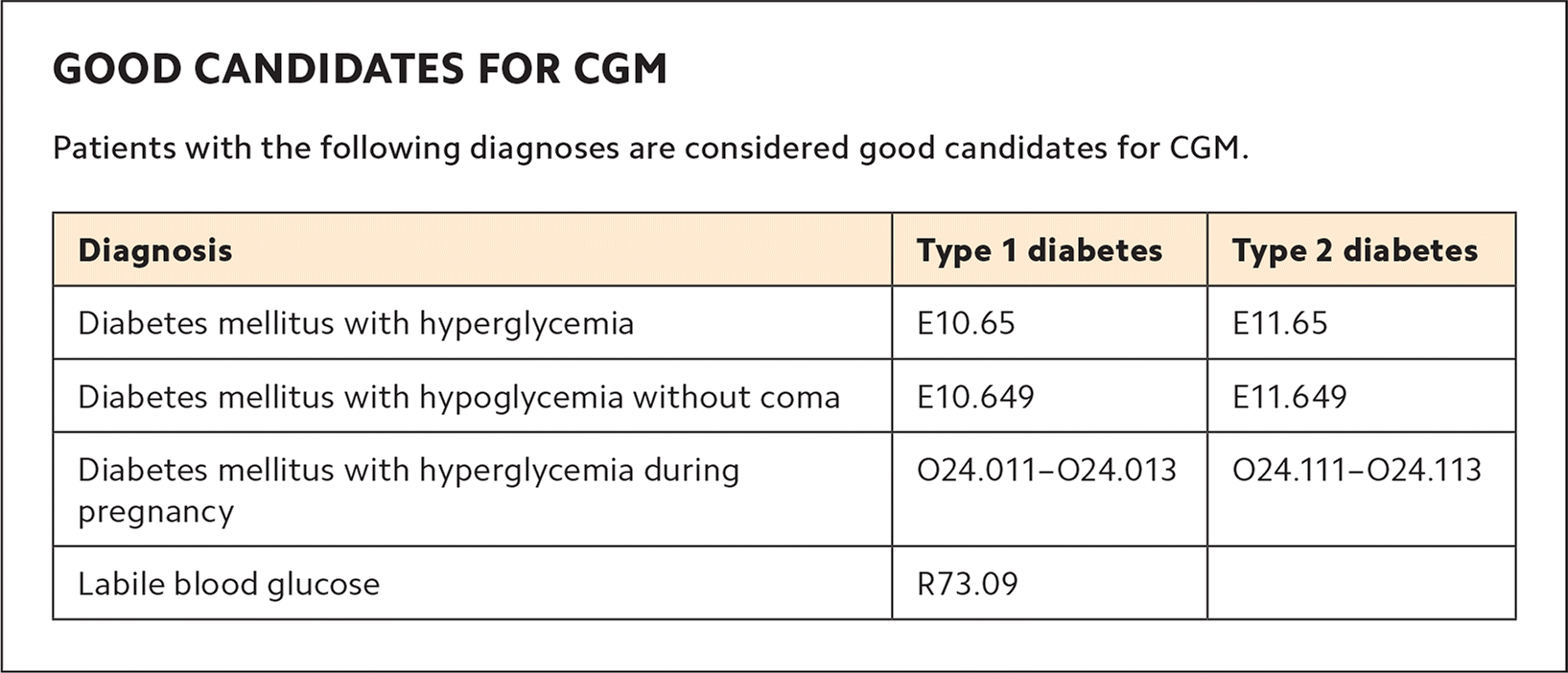
Select patients. Physicians and staff will need to learn to identify appropriate patients for CGM. Good candidates include those with uncontrolled diabetes, lack of hypoglycemia awareness, discordant A1C and finger-stick readings, dawn phenomenon, postprandial hyperglycemia, nocturnal hypoglycemia, suspected medication nonadherence, and special populations (e.g., chronic kidney disease or pregnancy). A spreadsheet with the names of potential CGM candidates should be distributed to all clinic physicians. (See “Good candidates for CGM.”)
| Diagnosis | Type 1 diabetes | Type 2 diabetes |
|---|---|---|
| Diabetes mellitus with hyperglycemia | E10.65 | E11.65 |
| Diabetes mellitus with hypoglycemia without coma | E10.649 | E11.649 |
| Diabetes mellitus with hyperglycemia during pregnancy | O24.011–O24.013 | O24.111–O24.113 |
| Labile blood glucose | R73.09 |
Establish a standardized workflow. The first step in the workflow is to obtain prior authorization before patients can have the sensors inserted. Professional CGM is payable by Medicare in all 50 states, but the Centers for Medicare & Medicaid Services has not established a national coverage policy. Coverage is instead determined by local Medicare contractors, and prior authorization criteria can vary between contractors. But based on our experience, patients are generally approved if they have uncontrolled diabetes, frequent testing (more than four times per day [Editor's note 9/18/2023: Medicare removed the four-fingersticks-per-day requirement in July 2021, expanding the number of patients who qualify for CGM]), unexplained highs or lows, or glucose readings discordant with their measured A1C. Patients who are pregnant, starting insulin for the first time, or starting an insulin pump are also usually approved. Most private insurers cover professional CGM for specific patient populations, often based on their type of diabetes and level of control. Check the written policies of payers in your area for their specific coverage criteria.
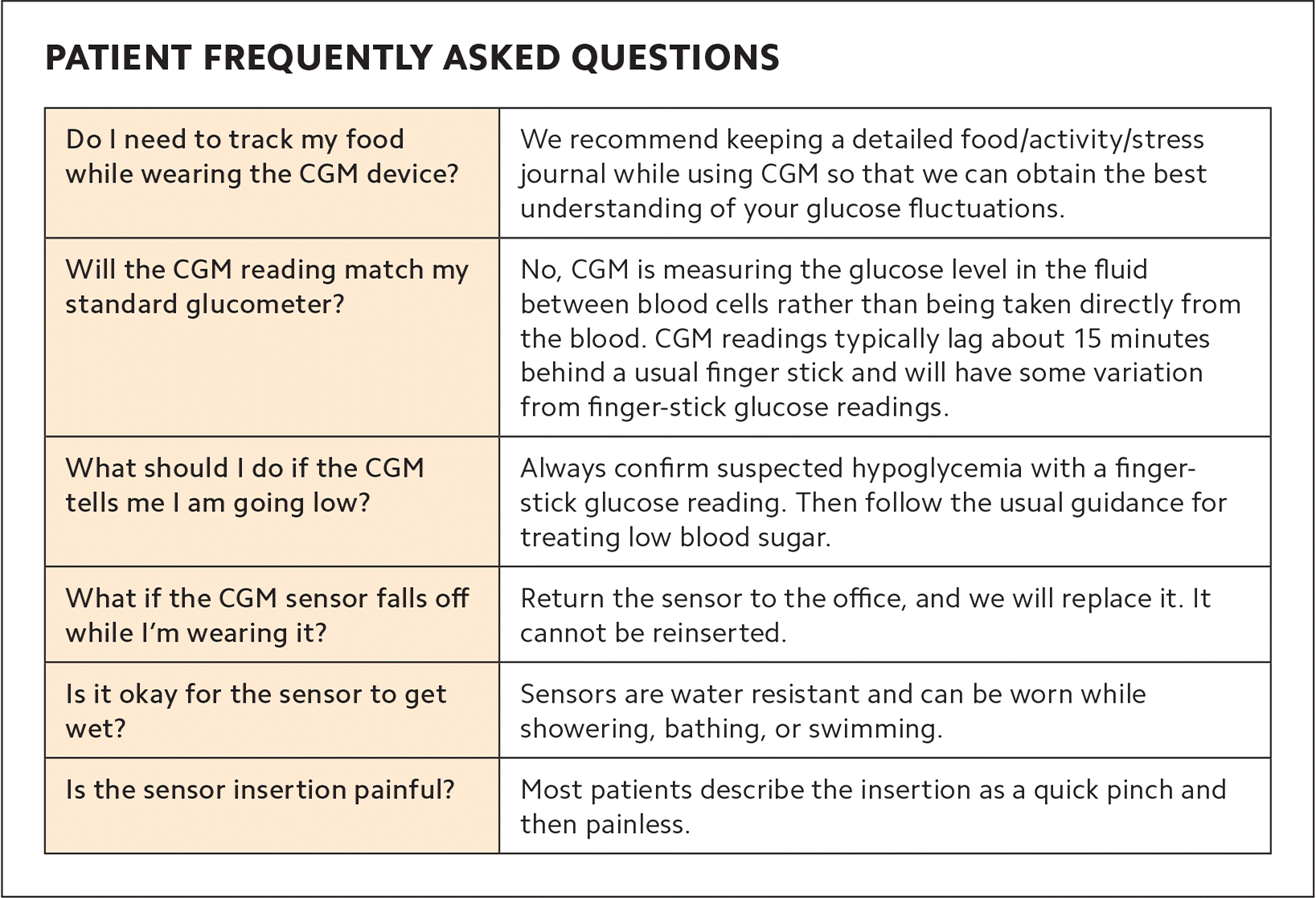
Once you have prior authorization from the patient's insurance carrier, you may insert the CGM sensor on the same day as an office visit or at a separate appointment. The insertion is a simple process using an auto-applicator. Trained staff can do the insertion and instruct the patient to keep a detailed food and activity record, all of which requires approximately 10 to 15 minutes of face time with the patient. (See “Patient frequently asked questions.”)
When the sensor's wear time expires, after six to 14 days, patients can easily remove the sensor by peeling off the adhesive. The patient then returns it to the clinic, either in person or via mail, for data downloading. The reader/receiver, which stays with the clinic in professional CGM, is used to extract the data from the sensor and upload it to a proprietary software program. All manufacturers offer cloud-based software to make the data upload and storage simple. Data reports can then be printed or sent electronically to the ordering physician for interpretation and therapeutic decision making. The patient should have a second office visit either in person or virtually to review and explain the reports. This could occur on the same day the sensor is returned or at a later date.
| Do I need to track my food while wearing the CGM device? | We recommend keeping a detailed food/activity/stress journal while using CGM so that we can obtain the best understanding of your glucose fluctuations. |
| Will the CGM reading match my standard glucometer? | No, CGM is measuring the glucose level in the fluid between blood cells rather than being taken directly from the blood. CGM readings typically lag about 15 minutes behind a usual finger stick and will have some variation from finger-stick glucose readings. |
| What should I do if the CGM tells me I am going low? | Always confirm suspected hypoglycemia with a finger-stick glucose reading. Then follow the usual guidance for treating low blood sugar. |
| What if the CGM sensor falls off while I'm wearing it? | Return the sensor to the office, and we will replace it. It cannot be reinserted. |
| Is it okay for the sensor to get wet? | Sensors are water resistant and can be worn while showering, bathing, or swimming. |
| Is the sensor insertion painful? | Most patients describe the insertion as a quick pinch and then painless. |
INTERPRETING CGM DATA
Professional CGM device manufacturers have tried to make CGM reports easy to understand and interpret. The summary reports vary slightly from one manufacturer to another, but all include a daily pattern report for each 24-hour period that the CGM sensor was worn, an overlay report that aggregates those daily glucose readings, and percentages of time above, below, or within the target glucose range (see “Sample CGM report”). Some of the devices also report hourly glucose statistics, mealtime insulin analyses, and other daily details. The summaries contain insights and suggested solutions based solely on the data, but reviewing the results with the patient can lead to better understanding of what they were doing at various times that might have affected glucose levels and enable meaningful conversations about how to improve glucose control.
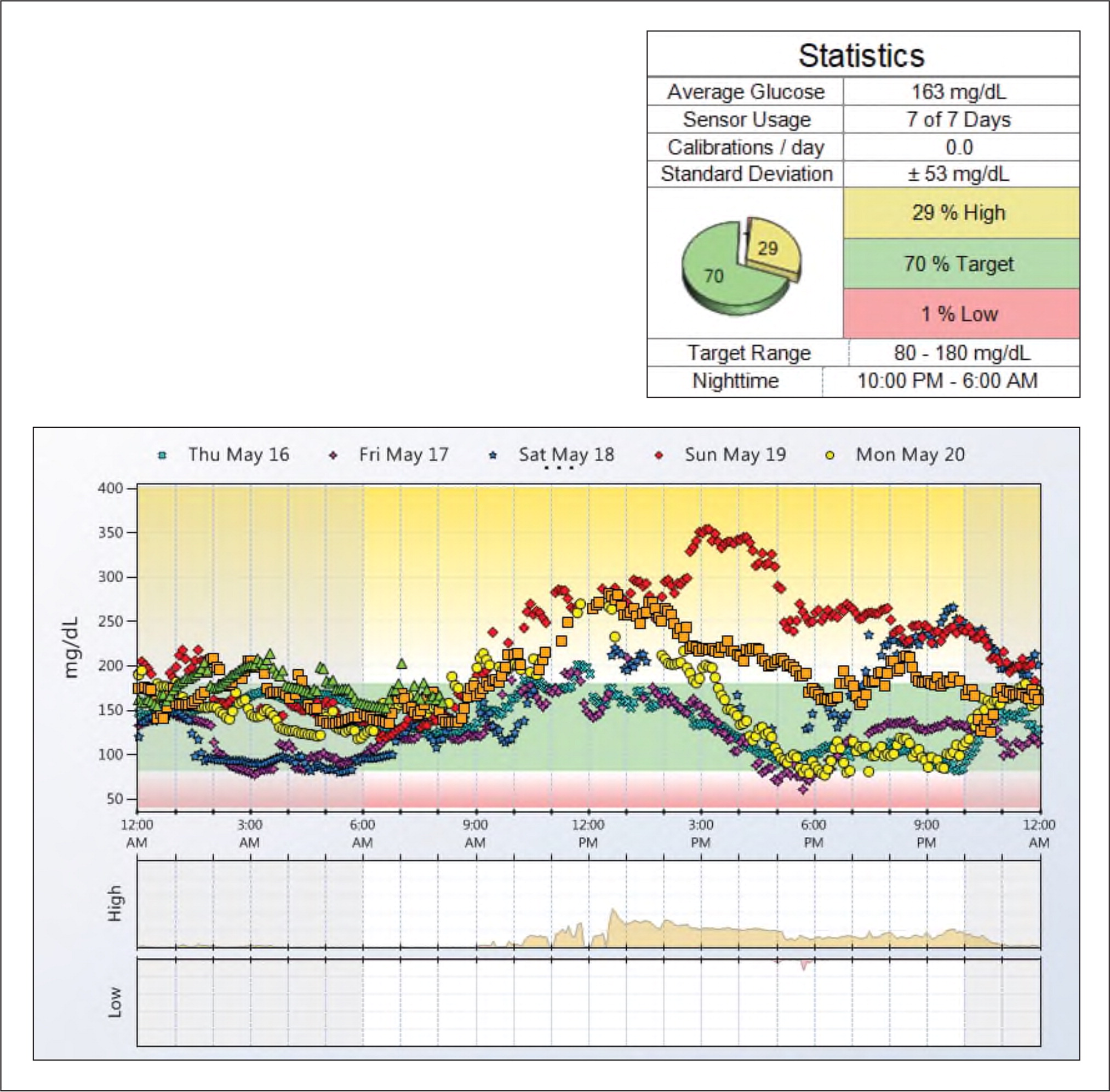
Developing a systematic or step-by-step method of reviewing the data will make interpretation more efficient.12,13 In our practice, we find it helpful to print the overlay report and use it to focus the patient conversation. Adding details such as typical mealtimes to correlate the glucose peaks and valleys can be especially useful. We also recommend asking patients about exercise habits or any other lifestyle factors that could affect the data. Write in the medications patients take and note the timing of insulin doses. Look for patterns of low glucose levels. Try to determine if these are outliers or common occurrences, and seek the patient's input on potential causes. Assess overnight glucose control. Take note of consistent areas of hyperglycemia or peaks and whether they occur pre- or post-prandially. Point out areas of consistently good glucose control, and help the patient recognize the source of these successes.
Once your observations are complete, develop an action plan with patients based on the problem areas identified. Make adjustments to their medication regimen or diet as needed. Give patients a copy of the report and add it to the electronic health record as well. Repeat the process as frequently as once a month to evaluate interventions.
PATIENT EXAMPLES
Here are three examples of how CGM data can lead to interventions that improve patients' health outcomes.
Patient 1 is a 73-year-old male with an initial A1C of 9.3% on insulin detemir and metformin who is prone to highs followed by lows, leading him to stop his mealtime insulin.
Data: CGM identified glucose level was higher than 180 in the period from midnight to 6 a.m. and had a large variance (100–350) during the day. This indicates poor food choices and evening snacking.
Interventions: The physician added a glucagon-like peptide-1 (GLP1) to his medication regime, and the dietitian increased education about portion size and carbohydrates. The patient was also able to identify and correct his low-fiber diet.
Result: His A1C subsequently improved to 6.2%.
Patient 2 is a 72-year-old female with initial A1C of 12.3% on 70/30 insulin isophane (NPH)/insulin regular and metformin.
Data: CGM showed that 94% of her glucose readings were in the target range, but she was having lows, particularly around lunchtime, and then over-correcting. Discussion with the patient revealed she was consuming too many carbs, such as an eight-ounce glass of orange juice, to correct for feeling low.
Interventions: The dietitian counseled her to eat lunch earlier and include protein, and provided education on a carb-controlled diet. The physician suggested adding five units of insulin lispro at dinner.
Result: Her A1C subsequently improved to 6.7%.
Patient 3 is a 69-year-old female with an A1C persistently over 10% for more than a year. She is taking 75 units of insulin glargine twice daily and eight units of insulin aspart three times daily with meals.
Data: CGM showed highs after breakfast and dinner, indicating large portions and poor food choices.
Interventions: The physician increased mealtime insulin and provided counseling on appropriate food choices.
Result: Her A1C subsequently improved to 9.3%.
CODING AND REIMBURSEMENT
The initial costs of adding CGM to a practice depend on the device. But a practice should be able to start a small CGM program with any of the three devices for less than $1,000 initially, and then ramp up from there as reimbursements begin.
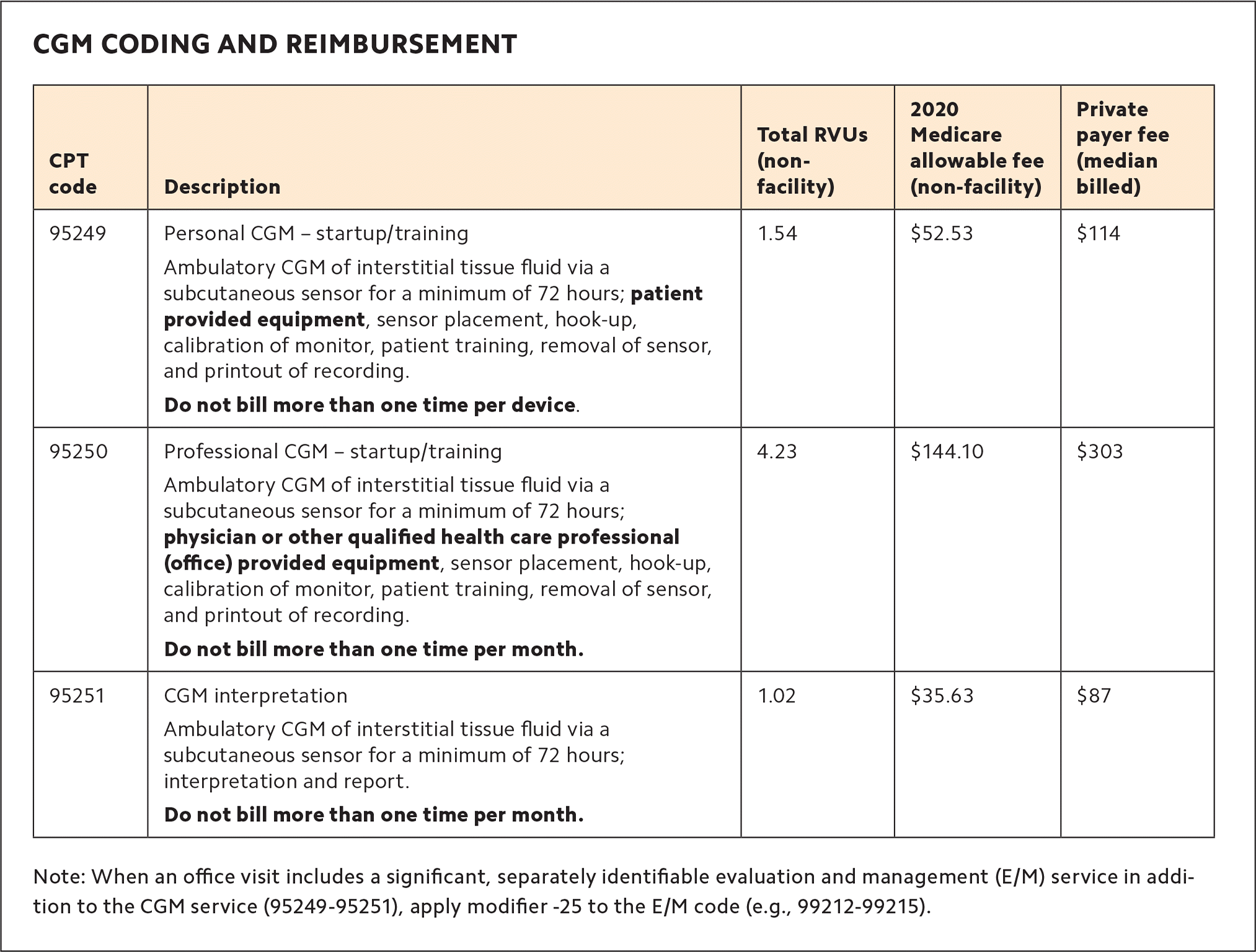
There are several CPT codes for CGM reimbursement:
Code 95250 is for training the patient, startup and calibration, sensor removal, and data download in a professional CGM program. This code may be billed by any qualified health professional (QHP).
Code 95249 is for training and startup of personal CGM, in the same way you would bill 95250 for professional CGM.
Code 95251 may be billed by a physician or QHP for the interpretation of the CGM report. This can be done with or without the patient in the office, but a face-to-face or virtual office visit is recommended to review the results and make therapy adjustments.
If an evaluation and management (E/M) service is performed on the same day as CGM services, report modifier -25 with the E/M code. (See “CGM coding and reimbursement.”)
The Medicare allowed charges for professional CGM (code 95250) are almost $150, and we have found that private payers in our area usually pay about twice as much. This allows a practice to quickly recoup startup costs and generate revenue.
Professional CGM advances the care of diabetes and generates revenue for a clinic. The increased data compared to finger-stick readings provides a helpful roadmap to guide discussions on dietary choices and to plan medication changes. A motivated and well-trained health care team will ensure the successful launch of a program.
| CPT code | Description | Total RVUs (non-facility) | 2020 Medicare allowable fee (non-facility) | Private payer fee (median billed) |
|---|---|---|---|---|
| 95249 | Personal CGM – startup/training Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; patient provided equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. Do not bill more than one time per device. | 1.54 | $52.53 | $114 |
| 95250 | Professional CGM – startup/training Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; physician or other qualified health care professional (office) provided equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. Do not bill more than one time per month. | 4.23 | $144.10 | $303 |
| 95251 | CGM interpretation Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; interpretation and report. Do not bill more than one time per month. | 1.02 | $35.63 | $87 |
