CDC Examines California Rotavirus Outbreaks in Post-vaccine Era
May 09, 2018, 01:57 pm Chris Crawford – Rotavirus disease, which causes severe diarrhea in young children, has substantially declined since the rotavirus vaccine was introduced in the United States in 2006.
However, according to the most recent data from the 2016 National Immunization Survey-Child, full rotavirus vaccine series completion (73 percent) in children ages 19-35 months was lower for this age group than completion of at least three doses of the diphtheria, tetanus and pertussis vaccine (95 percent), which is given on a similar schedule to the rotavirus vaccine.
The CDC said two rotavirus vaccines (Rotarix [two-dose series at ages 2 and 4 months]; RotaTeq [three-dose series at ages 2, 4 and 6 months]) are currently licensed for use in the United States. Both of these vaccines have demonstrated good field effectiveness (78 percent to 89 percent) against moderate to severe rotavirus illness, and their use has substantially reduced the prevalence of rotavirus in the United States.
Because rotavirus vaccination is the best way to reduce rotavirus disease in the United States, the CDC said efforts to increase rotavirus vaccination coverage need to continue.
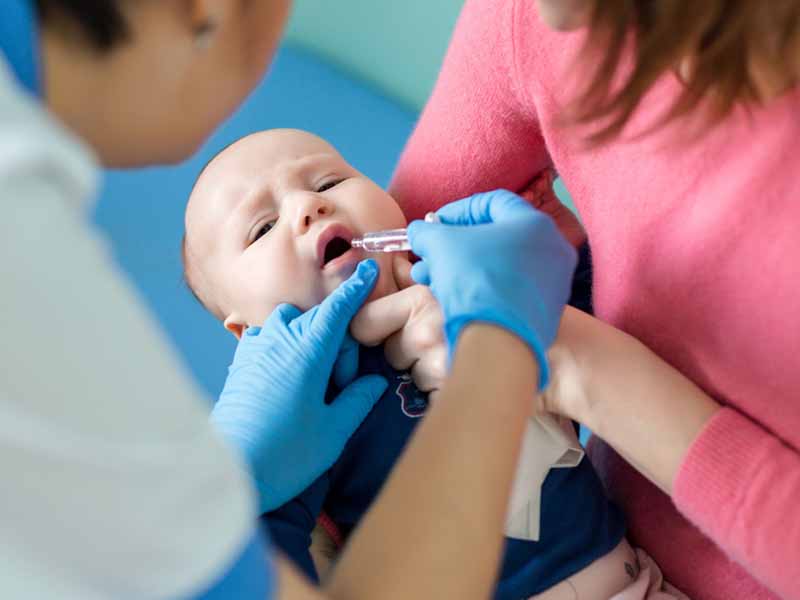
This is according to an April 27 Morbidity and Mortality Weekly Report (MMWR), in which the CDC examined three rotavirus outbreaks in California in 2017, and found mostly mild to moderate illness among vaccinated and unvaccinated children and adults. However, one unvaccinated child with underlying complications died.
The agency said the rotavirus outbreaks in California provide a view of typical outbreaks in the post-vaccine era, and noted that outbreaks may continue even with broader rotavirus vaccine coverage, as the immunization does not provide full protection, especially against mild disease.
Story Highlights
California Outbreaks
The first California outbreak examined occurred in late March 2017, when the Long Beach Department of Health and Human Services was notified of an outbreak of acute gastroenteritis (AGE) at a child care center.
By April 17, 2017, 27 cases of AGE among children and four cases among staff members were reported, with five secondary cases among household contacts recorded.
Among 31 patients for whom symptom information was available, 71 percent had diarrhea, 55 percent had vomiting, 42 percent reported abdominal cramps, 38 percent had fever and 13 percent reported nausea.
Patient ages ranged from 2-86 years (median age 4 years); three patients visited their primary care provider and no hospitalizations or deaths occurred.
Norovirus was initially suspected as the cause of this outbreak, but four stool specimens tested at the Long Beach Public Health Laboratory were norovirus-negative. Specimens were then sent to the California Department of Public Health (CDPH) Viral and Rickettsial Disease Laboratory (VRDL), a CaliciNet Outbreak Support Center, where they all tested positive by reverse-transcription polymerase chain reaction (RT-PCR) for rotavirus.
The California immunization registry indicated that 22 percent of the 27 children with rotavirus were vaccinated against it, including four who were fully vaccinated. However, the CDC said actual coverage might have been higher in this population because use of the registry isn't mandated and the child care center didn't require proof of rotavirus vaccination for enrollment.
The second outbreak happened in early April 2017, when the San Mateo County Division of Public Health, Policy and Planning was notified of an outbreak of AGE at an assisted living and memory care facility.
By April 10, 2017, nine cases had been reported, including four among residents and five among staff members. Symptom onset occurred from March 31 to April 6.
All nine patients had diarrhea, two reported abdominal cramps and one had vomiting.
Patient age ranged from 22-90 years (median age 47 years), with none of these patients eligible to receive rotavirus vaccine.
At least one patient sought primary care; no hospitalizations or deaths were reported.
As in the first outbreak, the CDC said norovirus was initially suspected, but two stool specimens tested at the county public health laboratory were norovirus-negative. These specimens were then sent to CDPH VRDL for additional testing, where they were both found to be rotavirus-positive by RT-PCR.
"The second outbreak, in an adult assisted living and memory care facility, demonstrated that rotavirus can and does cause illness in adult populations and can spread easily among adults living in close quarters, such as nursing homes," the MMWR said.
On May 1, 2017, the third outbreak was reported to the Santa Clara County Public Health Department as an outbreak of AGE at a subacute inpatient care facility for patients younger than 21 with complex medical needs.
During the outbreak, 24 of the 25 facility patients and three of 115 staff members were affected.
Symptom onset occurred from April 24 through May 17, 2017, with a median duration of symptoms of 7.5 days. Eighty-five percent of these patients had diarrhea; 56 percent had vomiting.
Patient age ranged from 6 months to 39 years (median age 2 years).
"Although most cases resolved without major complications, one child aged 22 months with preexisting respiratory failure died; the cause of death was attributed to rotavirus-induced dehydration," the report said. "This patient, as well as 16 others, had received no doses of rotavirus vaccine; three other patients had received a single dose."
The CDC said that although the facility didn't track the reasons for non-vaccination, records showed many of the children had been vaccinated according to delayed vaccination schedules and might have aged out of eligibility for rotavirus vaccination.
Laboratory testing by a gastroenteritis multi-pathogen PCR panel at a local hospital confirmed rotavirus in 11 of 14 samples; no other pathogens were detected.
Eight samples forwarded to CDPH VRDL were found to be PCR-positive for rotavirus, and five were then forwarded to CDC for genotyping.
Preventing Rotavirus
In addition to vaccinating against rotavirus, the CDC's public health recommendations to prevent rotavirus mirror those for norovirus.
The agency said hand hygiene, cohorting and isolation, and surface disinfection with appropriate products should be emphasized.
"Cleaning surfaces with soap and water followed by a five-minute application of 1,000-5,000 ppm chlorine solution (5-25 tablespoons [2.5-12.5 ounces] of household bleach [5.25 percent sodium hypochlorite] per gallon of water) or other disinfectant registered as effective against norovirus by the Environmental Protection Agency is appropriate for both pathogens," the MMWR said.
Long Beach Family Physician's Perspective
AAFP member Jeffrey Luther, M.D., is the director of health policy for the Memorial Family Medicine Residency Program in Long Beach, Calif. -- the area of the first outbreak described.
Luther told AAFP News that although he can't definitely say the cases of AGE he saw in patients around the time of the Long Beach outbreak were linked to rotavirus, he said the disease is always included in the differential diagnosis, particularly in the pediatric population.
"We treat plenty of children (and adults) with diarrheal illness, but for the mild- to moderate-severity cases, we treat what appears to be viral enteritis empirically without typically identifying the causative agent," he said. "Practically, in the outpatient setting, such illness is treated much the same regardless of pathogen (as the CDC article points out)."
For more-severe cases of AGE, Luther said his practice admits and manages its patients in a large children's hospital on its campus, where causative organisms are more commonly identified.
"For inpatients, identifying the cause is more routine, both to inform prognosis and to guide potential contacts," he said.
As for encouraging parents to immunize their children with the rotavirus vaccine, Luther said that although his community has its share of vaccine-hesitant parents, the rotavirus vaccine has been easier to sell than some other early childhood vaccines.
"I suspect this is in part because it's oral rather than injected, and perhaps more importantly, because most all parents can identify with diarrheal illness, while so many other vaccine-preventable diseases are less common or seem to be historic," he said. "The primary challenge (with the rotavirus vaccine) is the short age window for immunization, since many of our patients have delayed immunization, often related to sporadic coverage or access to care."
Luther said he describes to parents the rotavirus vaccine's safety and the contrasting severity of gastroenteritis, including the risk of hospitalization and death.
Finally, Luther said the approach to gain full rotavirus immunization coverage might need to be reimagined, as the obstacles the vaccine faces differ from those that limit the delivery of other childhood vaccines.
Related AAFP News Coverage
Academy, STFM Launch Shots by AAFP/STFM
(4/2/2018)