CDC Approves Second COVID-19 Vaccine
AAFP Offers Updated Guidance, Distribution Information
December 21, 2020, 10:25 am Michael Devitt — One week after the CDC officially approved the use of a vaccine candidate from Pfizer and BioNTech against the virus that causes COVID-19, the agency has given the same approval to a second vaccine candidate from Moderna Inc., providing family physicians and other health care professionals with another resource in the ongoing pandemic.
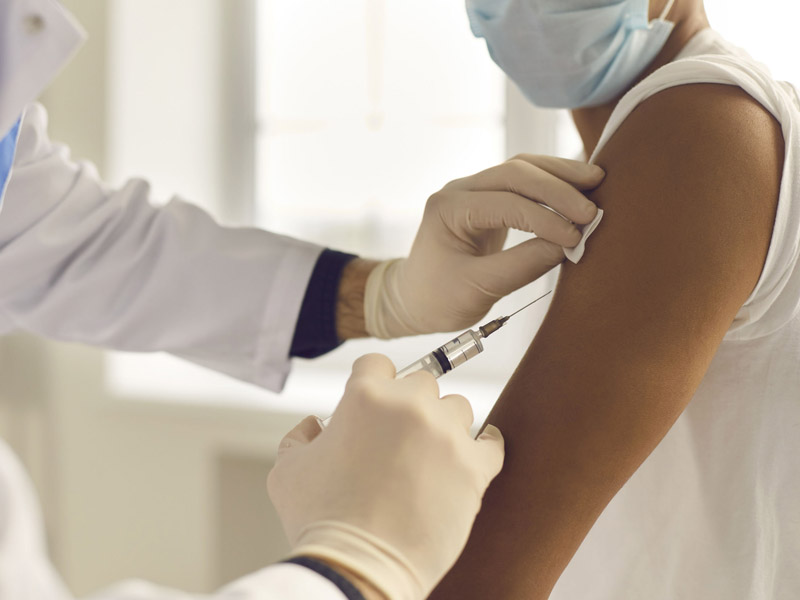
The vaccine candidate, mRNA-1273, received official approval from the CDC on Dec. 20, following an 11-0 vote (with three recusals) by the CDC’s Advisory Committee on Immunization Practices on Dec. 19 to recommend the Moderna vaccine for use in individuals 18 and older.
The ACIP’s full interim recommendation for use of the Moderna vaccine was published as a Morbidity and Mortality Weekly Report Early Release on Dec. 20.
The CDC’s actions were preceded by a favorable recommendation — 20-0 with one abstention — for the Moderna vaccine from the FDA’s Vaccines and Related Biological Products Advisory Committee at a Dec. 17 meeting. The FDA then issued an emergency use authorization for the vaccine the following day.
AAFP Approves ACIP Recommendations
The AAFP reviews all ACIP recommendations for approval through the Commission on Health of the Public and Science. As the Moderna vaccine will be distributed and administered to health care workers as early as this week, members of the commission’s executive committee reviewed the clinical trial data and the balance of benefits and harms presented by the ACIP. The board chair approved the executive committee’s recommendation for supporting ACIP’s 2020 interim recommendation for use of the vaccine under an EUA.
“We are pleased to have a second COVID-19 vaccine approved, allowing further progress to prevent COVID-19, control the global pandemic and protect those caring for patients and other health care workers,” said Julie Wood, M.D., M.P.H., the Academy’s senior vice president for research, science and health of the public. “The potential for increased accessibility in more communities is particularly important for family physicians and the patients we serve.”
STORY HIGHLIGHTS
Following the FDA’s authorization and the CDC’s recommendation, family physicians and other health care professionals may be able to start receiving the first distribution of the Moderna vaccine as early as this week. The Academy’s COVID-19 Vaccine Distribution page contains several resources for FPs to find their specific state and jurisdiction vaccine distribution plans.
On Dec. 20, the ACIP also discussed and voted to approve interim recommendations for the next phase of allocation for the COVID-19 vaccines. Phase 1b would prioritize persons aged 75 and older and frontline essential workers. Phase 1c would prioritize individuals aged 65-74, persons aged 16-64 with high-risk medical conditions, and other essential workers not captured in phase 1b.
Vaccine Comparison
While both of the approved vaccines have demonstrated more than 90% efficacy, there are small but important differences between the two that will likely affect how and where they will be distributed.
First, whereas the Pfizer vaccine must be stored at roughly minus 70 degrees Celsius, which requires specialty freezers, the Moderna vaccine can safely be stored at minus 15 degrees Celsius. This temperature can be achieved by most regular freezers, making the Moderna vaccine potentially more accessible for clinicians who practice in smaller facilities and less populated communities.
Second, the Pfizer vaccine can be refrigerated for up to five days after it is thawed. In contrast, the Moderna vaccine can last in a refrigerator for 30 days after thawing. In addition, unpunctured vials of the Moderna vaccine can be kept outside of a refrigerator at room temperature for up to 12 hours, which may increase options for administering the vaccine in the outpatient setting or other venues.
Third, while both vaccines are administered in a two-dose regimen, the recommended time between doses is 21 days for the Pfizer vaccine and 28 days for the Moderna vaccine.
Fourth, while the Pfizer vaccine received an EUA for use in individuals 16 and older, the Moderna vaccine’s EUA is for individuals 18 and older — an important consideration for parents of teenage children who are eligible to be vaccinated.
Additional Guidance
Based on reports of anaphylactic reactions in some people who have received a COVID-19 vaccine, the CDC published additional guidance for health care professionals on Dec. 19.
Among other things, the CDC recommends that anyone who has ever had a severe allergic rection to any ingredient in a COVID-19 vaccine should not receive that specific vaccine. Anyone who experiences a severe allergic reaction after getting the first dose of a COVID-19 vaccine should not receive the second dose.
The CDC also recommends that people who have previously had a severe allergic reaction to another vaccine or injectable therapy consult with their physician to determine whether they should receive a COVID-19 vaccine. People with a history of severe allergic reactions to other substances, such as certain foods, pet dander or latex, and people who might have a milder allergy to vaccines, may still get vaccinated.
More detailed information on the CDC's recommendations and on the potential management of anaphylaxis at sites where COVID-19 vaccines are administered is available online.
AAFP Resources
The Academy will continue to keep members aware of the latest news related to COVID-19.
The AAFP's COVID-19 page, which debuted in March, is regularly updated to give members information to support their practices and their patients.
In addition, resources related to vaccines will be posted on the AAFP COVID-19 Vaccine webpage and communicated to members as they become available.
The Academy is also providing resources through familydoctor.org to ensure that patients have the latest information on COVID-19 and that they learn about the safety and benefits of vaccines.