Pfizer COVID-19 Vaccine Now Authorized for 12- to 15-Year-Olds
May 13, 2021, 5:51 p.m. News Staff — On May 12, following a vote by the CDC’s Advisory Committee on Immunization Practices earlier in the day to recommend expanding use of the Pfizer-BioNTech COVID-19 vaccine to include children and adolescents aged 12 to 15 years, the agency officially adopted the advisory committee’s recommendation.
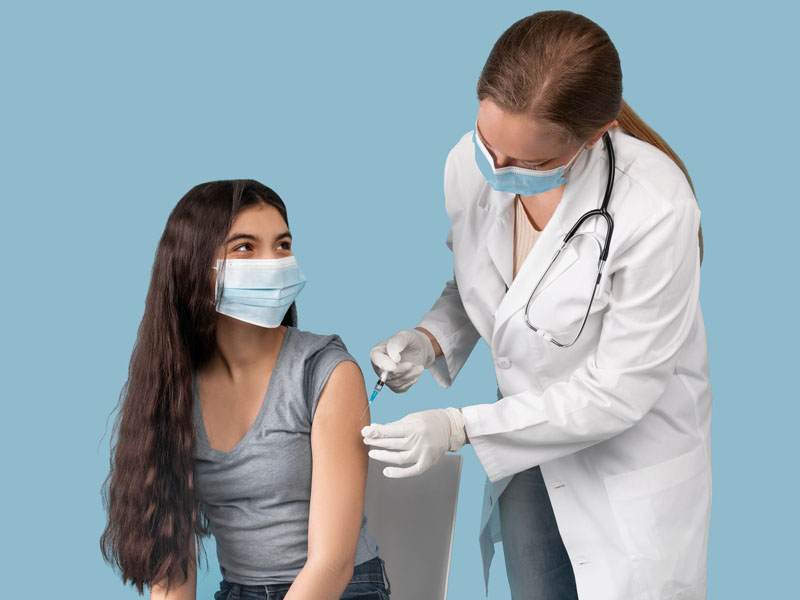
The AAFP has approved the recommendation following an expedited review of the evidence. The new recommendation means that family physicians and other health care professionals who have access to the Pfizer-BioNTech vaccine may begin administering it to patients in that age group immediately, as the dose for individuals aged 12 to 15 is the same as for those aged 16 and older.
“This week marks an important milestone as the first COVID-19 vaccine received emergency use authorization for children ages 12 to 15,” AAFP President Ada Stewart, M.D., of Columbia, S.C., said in a statement. “The American Academy of Family Physicians is encouraged that adolescents can now receive this safe and effective vaccine, as recommended by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.”
The CDC’s decision came two days after the FDA amended the emergency use authorization of the Pfizer-BioNTech vaccine to include individuals ages 12 to 15. The FDA originally issued an EUA for the vaccine on Dec. 11, 2020, for administration in individuals 16 and older.
Story Highlights
At an emergency meeting held virtually May 12, the ACIP reviewed data from a recently conducted phase 3 trial of the vaccine involving 2,260 adolescents. Trial results indicated that the Pfizer-BioNTech vaccine produced robust antibody responses and demonstrated 100% efficacy, with no cases of symptomatic COVID-19 or severe allergic reactions in adolescents who were fully vaccinated. Side effects in this age group were similar to those observed in older individuals, with pain at the site of injection, fatigue and headache the side effects most commonly reported.
The ACIP’s final vote to recommend use of the vaccine was 14-0, with one recusal.
Up Next
The CDC will be publishing updated clinical considerations for COVID-19 vaccines to include the new recommendation as well as updated language around co-administration of the COVID-19 vaccines with other recommended immunizations. The AAFP COVID-19 Vaccine webpage will be updated once that information is available.
Additionally, the AAFP is partnering with the CDC on a webinar for members on the latest recommendations for adolescents and clinical considerations for COVID-19 vaccines. The webinar, hosted and moderated by Stewart, is scheduled for May 17 at 7 p.m. CST and will be streamed across the AAFP’s Facebook and Twitter pages and the Academy’s YouTube channel. Sarah Mbaeyi, M.D., M.P.H., and Stephen Flores, Ph.D., from the CDC’s Vaccine Task Force will join Stewart to provide updates, and members will have an opportunity to ask questions. Look for more information on the Academy’s social media pages and in the AAFP COVID-19 Member Community.
What Family Physicians Can Do
In her statement, Stewart noted that family physicians serve as a primary source of childhood immunizations and other services. She also referred to a recently launched effort by the CDC in partnership with individual states to enroll FPs, pediatricians and others as COVID-19 vaccination providers to increase vaccine access in the coming weeks. Family physicians who are interested in providing the vaccine but have not enrolled can sign up via a CDC enrollment page.
“The AAFP is encouraged by President Biden’s intent to distribute vaccines directly to family medicine practices and we look forward to hearing more about the administration’s plan for implementation,” said Stewart. “This is a welcome change, as recent survey data from the Kaiser Family Foundation suggests patients would prefer to receive the vaccine from their own physician.”
In addition, Stewart encouraged FPs to speak with their patients about how to get the vaccine, and directed members to the familydoctor.org vaccines page for more information.
It should be noted that the Academy has a wide range of COVID-19 resources available for clinicians as well, including the COVID-19 Vaccine webpage, which is routinely updated to provide members with the latest news and information on the topic.