AAFP Signs Off on Pediatric COVID-19 Vaccine Recommendations
FDA, CDC, ACIP Approve Use of Pfizer-BioNTech Vaccine in Young Children
November 3, 2021, 4:25 p.m. News Staff — CDC Director Rochelle Walensky, M.D., on Nov. 2 endorsed a unanimous recommendation issued by the agency’s Advisory Committee on Immunization Practices earlier in the day to allow for the use of the Pfizer-BioNTech COVID-19 mRNA vaccine in children ages 5 to 11 years.
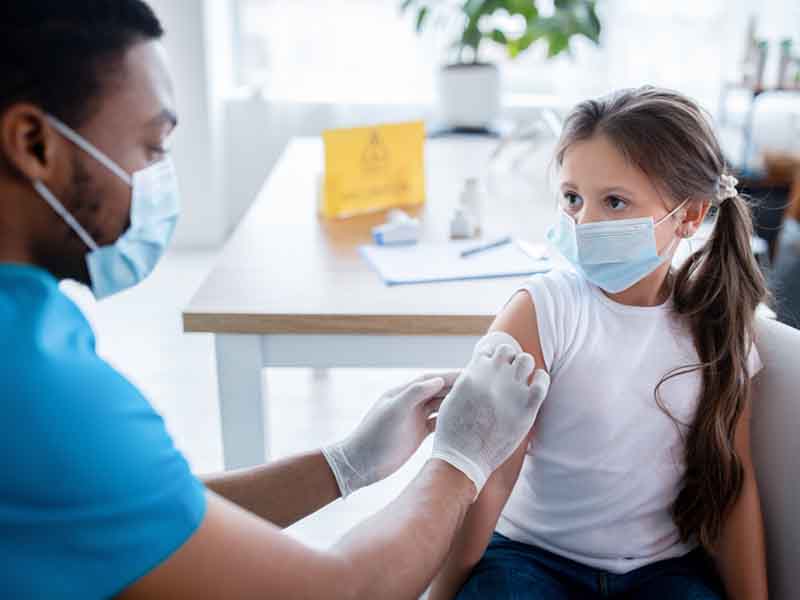
The AAFP approved the advisory committee’s recommendation after conducting an expedited review of the evidence, and has updated its COVID-19, COVID-19 Vaccine and COVID-19 Vaccine for Children and Adolescents webpages with the latest information.
Walensky’s endorsement clears the way for many family physicians and other health care professionals to begin administering the vaccine to children as early as this week.
In a White House briefing on Monday, White House COVID-19 response coordinator Jeff Zients said the pediatric vaccination program will be fully operational across the country by the week of Nov. 7. He added that the White House had procured 28 million pediatric doses of the Pfizer-BioNTech vaccine, enough to vaccinate every child ages 5 to 11 in the United States.
The AAFP’s approval, Walensky’s endorsement and the advisory committee’s vote followed the FDA’s Oct. 29 emergency use authorization for the Pfizer-BioNTech vaccine in this age group.
Story Highlights
AAFP President Sterling Ransone Jr., M.D., of Deltaville, Va., explained the importance of the decision and the role of family physicians in delivering the vaccine in a Nov. 2 statement.
“Family physicians play an instrumental role in caring for, counseling, and immunizing children and their families,” Ransone said. “We provide routine immunizations for children, adolescents, pregnant individuals and other adults, and have trusting relationships with our patients, understand their unique health needs, and stand ready to counsel them about and administer COVID-19 vaccines.”
“Ensuring children have equitable access to a safe, effective vaccine is critical to ending this pandemic,” Ransone added. “The AAFP urges federal, state and local public health officials to ensure vaccines are swiftly distributed to primary care physicians.”
About the Vaccine
The Pfizer-BioNTech COVID-19 vaccine for children is administered as a two-dose primary series, with the second dose given three weeks after the first.
Whereas the dose used for individuals ages 12 years and older is 30 micrograms, the pediatric dose is 10 micrograms.
To reduce the chances of children receiving too much vaccine, the pediatric vaccine will be shipped in vials featuring orange caps, with orange stripes on the vaccine label and the product packaging as additional safety measures. The version of the vaccine for all other individuals has been shipped in vials with purple caps.
Clinical trial results showed that the lower dose of vaccine induced a robust antibody response with minimal side effects. There were no serious adverse events related to the vaccine observed in the study and no cases of myocarditis/pericarditis were observed. Additionally, the lower dose was 90% effective at preventing symptomatic COVID-19.
At the Nov. 2 ACIP meeting, committee members voted 14-0 to recommend use of the vaccine in children ages 5 to 11 years.
Several liaisons from primary care organizations, including the AAFP, offered support for the proposed recommendation prior to the committee’s vote. Pamela Rockwell, D.O., of Ann Arbor, Mich., reiterated the AAFP’s support for all children to be eligible to receive the vaccine as protection from the very real and significant harms of COVID-19.
“Speaking as a family physician with 30 years of clinical experience treating both children and adults in my practice, I have seen the devastating effects of COVID-19 infection, not only in my patients who have died from the disease, but also in those who have survived and now suffer from long-term effects of COVID-19; they suffer physical, mental and emotional effects of long-haul COVID. As we have seen in the presentations today, long-haul COVID also occurs in children, and data are still emerging,” Rockwell said.
“Vaccination of children ages 5 to 11 years will not only help prevent COVID-19 infection and serious consequences of infection in this age group, but it will also help children emotionally and socially to improve their development,” she added. “It will help reduce inequities in the impact of COVID-19 on our population, reduce the need for future school closures, disruptions and obligatory times of quarantine, allowing sports, after-school and other school-based and social activities to occur without risk and anxiety, and help improve our children’s quality of life, not just prevent COVID-19 illness and death in children and adults around them.”
More AAFP Resources
The Academy has routinely provided information on vaccines for children and adolescents along with resources from the CDC and other agencies to assist family physicians in preparing to administer the pediatric COVID-19 vaccine.
Staff and medical experts at the Academy have continued to update resources and content on AAFP.org and familydoctor.org to keep members apprised of the latest information. The Academy’s COVID-19 and COVID-19 Vaccine webpages contain the latest data on the pandemic and the vaccines used to fight COVID-19, along with information on recent and upcoming FDA and ACIP meetings, educational materials for clinicians and patients, advocacy, and more.