
Am Fam Physician. 1998;57(4):719-726
See related patient information handout on coronary artery disease, written by the authors of this article.
The continued occurrence of occupational lead overexposure and lead poisoning in the United States remains a serious problem despite awareness of its adverse health effects. Lead exposure is arguably the oldest known occupational health hazard. It is a particularly insidious hazard with the potential for causing irreversible health effects, including hypotension, central nervous system problems, anemia and diminished hearing acuity before it is clinically recognized. Scientific evidence of subclinical lead toxicity continues to accumulate, making further reduction in workplace exposure, regular screening, and earlier diagnosis and treatment of critical importance in the prevention of this occupational hazard. For the most part, the diagnosis of lead poisoning in the adult worker is based on the integration of data obtained from the history, a physical examination, laboratory tests and tests of specific organ function. A blood lead level of 40 μg per dL (1.95 μmol per L) or greater requires medical intervention; a level of 60 μg per dL (2.90 μmol per L) or three consecutive measurements averaging 50 μg per dL (2.40 μmol per L) or higher indicate the necessity for employee removal. The decision to initiate chelation therapy is not based on specific blood lead levels but depends on the severity of clinical symptoms.
Occupational lead poisoning has been a recognized health hazard for more than 2,000 years. Characteristic features of lead toxicity, including anemia, colic, neuropathy, nephropathy, sterility and coma, were noted by Hippocrates and Nikander in ancient times, as well as Ramazzini and Hamilton in the modern era.1 Physicians have gained an extensive understanding of the causes, the clinical presentations and the means of preventing lead poisoning. However, it remains one of the most important occupational and environmental health problems.2
Lead serves no useful biologic function in the human body. Over the past several years, concern has increased over the health effects of low-level lead exposure and the “normal” body burden of lead. In the occupational setting, the present “no-effect” level for lead exposure is currently being reevaluated as more sensitive measures of the physiologic effects of lead are made available through clinical investigations.3 Based on current knowledge of the health effects of lead in adults, the U.S. Public Health Service has declared a health objective for the year 2000: the elimination of all exposures that result in blood lead concentrations greater than 25 μg per dL (1.20 μmol per L) in workers.4
Occupational Exposure and the OSHA Lead Standard
Lead and lead compounds play a significant role in modern industry, with lead being the most widely used nonferrous metal.5 A wide variety of industrial populations is at risk of occupational exposure to lead (Table 1). According to estimates made by the National Institute of Occupational Safety and Health (NIOSH), more than 3 million workers in the United States are potentially exposed to lead in the workplace. Occupational exposure to lead in general industry is regulated by the 1978 Occupational Safety and Health Administration (OSHA) Lead Standard. The general industry standard specifies permissible limits on airborne lead exposure, as well as blood lead levels (Table 2). A construction standard, recently extended to cover workers in the construction industry, has slight differences in detail. However, enforcement of both standards is inadequate, and significant occupational exposure remains widespread.6
| Battery manufacturing |
| Chemical industry |
| Construction workers |
| Demolition workers |
| Firing-range instructors |
| Foundry workers |
| Gas-station attendants |
| Gasoline additives production |
| Jewelers |
| Lead miners |
| Lead smelters and refiners |
| Pigment manufacturing |
| Pipe fitters |
| Plastics industry |
| Pottery workers |
| Printers |
| Radiator repair |
| Rubber industry |
| Soldering of lead products |
| Solid waste production |
| Stained-glass makers |
| Welders |
For workers in the United States who are covered by the OSHA lead standard, a detailed medical examination is delineated under specific conditions3 (Table 3). Any general industry worker (i.e., battery, foundry, smelting, mining, glass, ceramics) found to have a single blood lead level of 60 μg per dL (2.90 μmol per L) or greater, or an average blood lead level of 50 μg per dL (2.40 μmol per L) or greater must be removed from the high-exposure job (termed “medical removal protection”). The “removed” worker should subsequently receive more frequent medical evaluation and blood testing (Table 4). A worker is not allowed to return to a job with the potential for high lead exposure until his or her blood lead level has fallen below 40 μg per dL (1.95 μmol per L) on two successive tests.6
| Detailed medical and occupational history | |
| Particular attention directed to lead exposure history (occupational and nonoccupational); personal and workplace hygiene; history of gastrointestinal, hematologic, renal, reproductive and neurologic disorders | |
| Physical examination | |
| Particular attention directed to neurologic and hematologic abnormalities; pulmonary status should be evaluated in workers required to wear respiratory protective devices. | |
| Blood pressure measurement | |
| Blood testing | |
| Blood lead level | |
| Zinc protoporphyrin or free erythrocyte protoporphyrin level | |
| Hemoglobin, hematocrit and peripheral smear | |
| Serum creatinine level | |
| Urinalysis with microscopic examination | |
| Supplementary laboratory tests as deemed clinically indicated | |
| note: Medical examinations are required in the following circumstances: | |
| (1) Yearly, if any blood level exceeds 40 μg per dL (1.95 μmol per L) | |
| (2) Before assignment in an area where air lead levels are at or above the action level | |
| (3) If a worker exhibits signs or symptoms of lead intoxication. | |
| A. Blood lead levels requiring employee removal. (Level must be confirmed with second follow-up level within 2 weeks of first report.) | ||
| 1. ≥60 μg per dL (2.90 μmol per L) or average of last three samples or all blood samples over previous 6 months (whichever is a longer time period) is 50 μg per dL (2.40 μmol per L) or greater unless last blood sample is 40 μg per dL (1.95 μmol per L) or less | ||
| B. Frequency with which employees exposed to action level of lead (30 μg per m3 TWA) must have blood lead level checked. Zinc protoporphyrin test is also strongly recommended at each occasion that a blood lead level is obtained: | ||
| 1. Last blood lead level is less than 40 μg per dL (1.95 μmol per L) | ||
| a. Every 6 months | ||
| 2. Last blood level is between 40 μg per dL (1.95 μmol per L) and level requiring medical removal (see A above) | ||
| a. Every 2 months | ||
| 3. Employees removed from exposure to lead because of an elevated blood lead level | ||
| a. Every month | ||
| C. Permissible airborne exposure limit for workers removed from work due to an elevated blood lead level (without regard to respirator protection) | ||
| 1. 30 μg per m3 per 8 hour TWA | ||
| D. Blood lead level confirmed with a second blood analysis, at which employee may return to work | ||
| 1. ≤40 μg per dL (1.95 μmol per L) | ||
A physician may also remove a worker with a lower blood lead level if risk of health impairment is suspected and may restrict the worker from return to exposure until medically approved.3 To prevent workers with elevated blood levels from being economically penalized during medical removal protection, the OSHA standard requires that these workers must continue to receive their full rate of pay (termed “pay rate retention”) while temporarily working in a less hazardous job.6
The mere presence of lead in the workplace does not necessarily signify a potential risk of poisoning. Lead is considered a hazard in the workplace primarily depending on the generation of respirable (below 5 μm) lead fumes or lead-containing dust particles in the work-room atmosphere. Consequently, no simple rule of thumb exists for categorizing different occupations into “more” or “less” hazardous classifications, alathough experience has shown that some types of work are indeed more dangerous than others (Table 1). Determining the magnitude of risk in a particular work process should always include a review of the process itself (does potential exposure involve dust, fumes or aerosolized particles?), the adequacy of abatement employed (local and general ventilation) and the general hygienic level of the workplace itself.2
Toxicology
Absorption
Lead is absorbed primarily through the respiratory and gastrointestinal systems, with the former being the more important route of entry in occupational exposures. Cutaneous absorption of inorganic lead is negligible. However, organic lead compounds, because of their lipid solubility, are readily absorbed through intact skin.5
Respiratory lead absorption is primarily dependent on particle size; solubility, respiratory volume and physiologic interindividual variation are less important factors. The percentage of inhaled lead reaching the bloodstream is estimated to be 30 to 40 percent.2
Gastrointestinal absorption of lead is lower in adults than in children, with an estimated 10 to 15 percent of lead in an adult's diet absorbed gastrointestinally. The degree of lead absorption is increased considerably with fasting or in persons whose diet is deficient in calcium, iron, phosphorus or zinc.5
Distribution
After lead is absorbed into the bloodstream, through either ingestion or inhalation, most of it is carried, bound, to erythrocytes. The freely diffusible plasma fraction is distributed extensively throughout tissues, reaching highest concentrations in bone, teeth, liver, lungs, kidneys, brain and spleen.2 Lead in blood has an estimated half-life of 35 days, in soft tissue 40 days and in bone 20 to 30 years.7 Inorganic lead does not undergo any metabolic transformation or digestion in the intestines, or detoxification in the liver.5
With chronic exposure over a long period of time, most absorbed lead ends up in bone. Lead, it appears, is substituted for calcium in the bone matrix. This is not known to cause any deleterious effect on bone itself. Bone storage likely acts as a “sink,” protecting other organs while allowing chronic accumulation. The lead that accumulates in the bone ultimately provides a source for remobilization and continued toxicity after exposure ceases.8 The total bodily content of lead is called the body burden; in a steady state, about 90 percent of the body burden is bound to bone.2
Excretion
Although lead is excreted by several routes (including sweat and nails), only the renal and gastrointestinal pathways are of practical importance. In general, lead is excreted quite slowly from the body (with the biologic half-life estimated at 10 years). Since excretion is slow, accumulation in the body occurs easily.2
Clinical Effects In Adults
Acute Inorganic Lead Toxicity
Excessive occupational exposure to lead over a brief period of time can cause a syndrome of acute lead poisoning. Classic clinical findings in this syndrome include abdominal colic, constipation, fatigue and central nervous system dysfunction. With even greater doses, acute encephalopathy with coma and convulsions may occur. In milder exposures, headaches and personality changes may be the only signs of neurologic toxicity6 (Table 5). 9
| Mild | Moderate | Severe |
|---|---|---|
| Myalgias | Headache | Encephalopathy |
| Irritability | Tremor | Motor neuropathy |
| Paresthesias | Vomiting | Seizures |
| Mild fatigue | General fatigue | Coma |
| Intermittent abdominal pain | Diffuse abdominal pain | Abdominal colic |
| Lethargy | Weight loss | Lead lines |
| Loss of libido | Oliguria | |
| Constipation |
Chronic Inorganic Lead Toxicity
Chronic toxicity is an insidious illness with protean manifestations.3,6 Symptoms may include arthralgias, headache, weakness, depression, loss of libido, impotence and vague gastrointestinal difficulties. Late effects may include chronic renal failure, hypertension, gout and chronic encephalopathy.6
Organ System Dysfunction
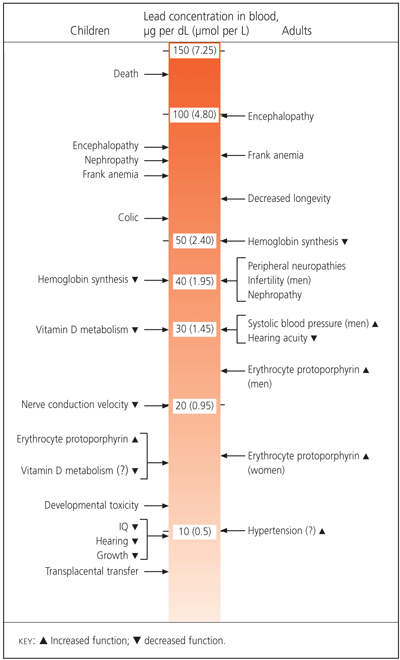
Lead toxicity can be manifested clinically in multiple organs. Specific organ system dysfunction includes the central and peripheral nervous system, and renal, hematologic, gastrointestinal and reproductive systems. Figure 1 provides a summary of the organ-specific effects associated with the lowest observable lead levels in the adult worker and, for comparison, in children.9
Diagnosis
Arriving at a diagnosis of lead poisoning in an adult requires a high index of suspicion and a careful history. The infrequency of classic diagnostic signs and the nonspecific nature of the symptoms frequently contribute to misdiagnosis.8
The diagnosis of inorganic lead intoxication in adults requires the demonstration of excess lead absorption, documentation of impairment in an organ system consistent with the effects of lead, and exclusion of other causes of disease. At present, the blood level concentration is the single best indicator of recent, acutely elevated lead absorption (Table 6). This level provides good information on lead absorption in persons with relatively brief exposure, such as construction workers or new entrants into the lead industry. The blood lead level rises rapidly within hours after an acute exposure and remains elevated for several weeks.6
Several attempts have been made over the years to relate blood lead levels to adverse health effects. It is not possible to determine a precise blood lead level below which symptoms never occur or a blood lead level at which symptoms are always reported. Individual susceptibility should always be recognized.5 Moreover, exposure to lead does not protect the worker from incurring other, unrelated diseases with signs and symptoms similar to those occurring in lead toxicity.
A zinc protoporphyrin or free erythrocyte protoporphyrin level reflects the toxic effect of lead on the erythrocytic enzyme ferrochelatase. Zinc protoporphyrin levels usually begin to rise in adults when the blood lead level exceeds 30 to 40 μg per dL (1.45 to 1.95 μmol per L). Once elevated, zinc protoporphyrin levels tend to remain above background level for several months. Since the zinc protoporphyrin level remains elevated long after exposure has ceased and the blood lead level has fallen, this test does not discriminate between recent and past exposure. It is only of ancillary value in evaluating occupational lead exposure.6 However, by obtaining both a blood lead level and a zinc protoporphyrin level, sufficient information is provided to quantitate the severity of the effect due to lead toxicity and the approximate chronology of the lead exposure (Table 7).2
A most difficult task is to either establish or exclude past lead exposure as the etiology of a current disease. Beyond several years since the last known lead exposure, the decision may be difficult, especially when all functional tests are now normal. One way to show a relationship between past lead exposure and present illness is to demonstrate an abnormally high body burden of lead. This is best done by measuring the excretion of lead in urine after provocation with edetate calcium disodium (calcium EDTA; Calcium Disodium Versenate) or another chelating agent. This is a reliable way to demonstrate an increased body burden with previous high-level exposures. However, it is important to recognize that this test indicates only past lead exposure, not past lead poisoning.2
In the future, the x-ray fluorescence method of analyzing lead content of bone may replace the EDTA chelation challenge as the gold standard of cumulative absorption. The measurement of bone lead content by x-ray fluorescence offers a noninvasive, relatively rapid approach to the assessment of body lead burden, with minimal radiation exposure (approximately one-tenth the radiation of a dental x-ray.)6 As x-ray fluorescence becomes more readily available, its safety, specificity and simplicity should make it an important alternative to chelation testing for the monitoring of lead burden for workers with documented chronic exposure.10
The clinical symptoms previously discussed are an important aid in diagnosis and should always be explored. Since many of the neurologic and gastrointestinal symptoms are non-specific, unless the examining physician is aware of the history of lead exposure, the diagnosis of lead toxicity may be easily overlooked. With improved control of the work environment resulting in lower levels of lead exposure, occupational lead poisoning is now usually characterized by subtle, nonspecific symptoms. Therefore, the diagnosis is typically made using laboratory tests along with a careful clinical evaluation.5
Treatment
In all cases of suspected lead intoxication in adults, the first step in management should be removal of the individual from the exposure.3,5,6 A physician who believes his or her patient has occupational lead poisoning should report the case to the local health department. Currently, 27 states require laboratory reporting of elevated lead levels under the Adult Blood Lead Epidemiology and Surveillance (ABLES) program administered by NIOSH (Table 8).Whether discontinuation of exposure is sufficient treatment or chelation therapy should be administered depends on the blood lead concentration, the severity of clinical symptoms, the biochemical and hematologic abnormalities, and the nature of the exposure. It is not recommended that specific blood lead concentrations be used to determine when treatment with a chelating agent is indicated. As a general rule, however, such a level is usually well above 80 μg per dL (3.85 μmol per L), which is also the level frequently associated with more severe symptoms.5 The primary indication for treatment of adults is brief, high-level exposure causing acute manifestations.3
The use of chelation is not generally indicated in cases of long-term occupational exposure. The OSHA Lead Standard specifically prohibits “prophylactic” chelation for the prevention of elevated blood lead levels.2 In prophylactic chelation, chelating or similarly acting drugs are used routinely to prevent elevated blood levels in workers who are occupationally exposed to lead or to lower blood lead levels to predesignated concentrations thought to be safe.11 In patients with ongoing lead exposure, chelation therapy is not considered appropriate medical treatment.12 Chronic administration of oral agents to workers who continue to be exposed to unacceptably high levels in their workplace is a technique that has been used by employers in the past and is considered inappropriate.6
The OSHA standard allows the use of “therapeutic” or “diagnostic” chelation only if administered under the supervision of a licensed physician in a clinical setting in conjunction with thorough and appropriate medical monitoring.11 When treatment with a chelating agent is indicated, edetate calcium disodium is often the drug of choice. Dimercaprol (BAL in Oil) and penicillamine (Cuprimine), popular agents in the past, are used less frequently today.5
The role of the new oral agent succimer (Chemet) in the treatment of adults has not been determined; it is currently approved by the U.S. Food and Drug Administration for treatment in children only.13 Although isolated reports document effectiveness of succimer in treating adults, no clinical trials with this agent for treatment of adult lead toxicity have been reported.14 The efficacy of chelating agents in treating patients with subtle neurologic and renal abnormalities has not yet been fully studied13 and is therefore not indicated.
It is essential to remember that the chelating agents themselves may have significant adverse effects, which represent a risk apart from the lead toxicity. Treatment with these agents usually represents a failure in preventing lead overexposure of the patient and should initiate investigation of other workers at risk. Physicians contemplating chelation therapy for treatment of lead poisoning are advised to consult with colleagues experienced with use of up-to-date treatment protocols in view of the lack of clear and consistent guidelines addressing the issue of when to chelate in such cases.12
Prevention
The first line of defense in the elimination of occupational lead poisoning is primary prevention—actions taken to prevent overexposure. Primary prevention is best achieved through the use of engineering controls, personal protective equipment and good work practices (Table 9). The family physician can have the greatest impact on prevention through worker education and instruction in proper personal hygiene techniques.
Following identification of a case of lead poisoning, preventive strategies should begin as worker removal and possible treatment are initiated. A single case of occupational lead poisoning may represent a “sentinel health event,” thereby serving as a warning of potential overexposure of other workers. With the assistance of industrial hygiene experts, the patient's workplace should be investigated for possible additional cases and causes. State health departments should be notified with possible referral to an occupational medicine specialist if questions remain regarding treatment protocol, worker disposition and work-place evaluation. Excellent resources that can assist the family physician in understanding these decisions are readily available and are listed in Table 10.
| Code of Federal Regulations | http://law.house.gov/cfr.htm |
| Duke University Occupational and Environmental Medicine | http://dukeoccmed.mc.duke.edu/ |
| CDC Homepage | http://www.cdc.gov/ |
| OSHA Regulations | http://www.osha.gov/SLTC/lead/ |
| EPA Homepage | http://www.epa.gov/ |
| NIOSH Homepage | http://www.cdc.gov/niosh/homepage.html |
| Envirolink | http://envirolink.org |