
Am Fam Physician. 1999;59(9):2565-2574
An increasing number of new and improved vaccines to prevent childhood diseases are being introduced. Combination vaccines represent one solution to the problem of increased numbers of injections during single clinic visits. This statement provides general guidance on the use of combination vaccines and related issues and questions. To minimize the number of injections children receive, parenteral combination vaccines should be used, if licensed and indicated for the patient's age, instead of their equivalent component vaccines. Hepatitis A, hepatitis B, and Haemophilus influenzae type b vaccines, in either monovalent or combination formulations from the same or different manufacturers, are interchangeable for sequential doses in the vaccination series. However, using acellular pertussis vaccine product(s) from the same manufacturer is preferable for at least the first three doses, until studies demonstrate the interchangeability of these vaccines. Immunization providers should stock sufficient types of combination and monovalent vaccines needed to vaccinate children against all diseases for which vaccines are recommended, but they need not stock all available types or brand-name products. When patients have already received the recommended vaccinations for some of the components in a combination vaccine, administering the extra antigen(s) in the combination is often permissible if doing so will reduce the number of injections required. To overcome recording errors and ambiguities in the names of vaccine combinations, improved systems are needed to enhance the convenience and accuracy of transferring vaccine-identifying information into medical records and immunization registries. Further scientific and programmatic research is needed on specific questions related to the use of combination vaccines.
The introduction of vaccines for newly preventable diseases poses a challenge for their incorporation into an already complex immunization schedule. To complete the 1999 Recommended Childhood Schedule in the United States,1,2 a minimum of 13 separate injections are needed to immunize a child from birth to age six years, using vaccines licensed in the United States as of April 10, 1999. During some office or clinic visits, the administration of three or four separate injections can be indicated.
Combination vaccines merge into a single product antigens that prevent different diseases or that protect against multiple strains of infectious agents causing the same disease. Thus, they reduce the number of injections required to prevent some diseases. Combination vaccines available for many years include diphtheria and tetanus toxoids and whole-cell pertussis vaccine (DTwP); measles-mumps-rubella vaccine (MMR); and trivalent inactivated polio vaccine (IPV). Combinations licensed in recent years in the United States include diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP),3–6 DTwP- Haemophilus influenzae type b (Hib) vaccine (DTwP-Hib),7,8 DTaP-Hib9 (As of April 10, 1999, DTaP-Hib vaccine was licensed only for the fourth dose recommended at age 15 to 18 months in the vaccination series.), and Hib-hepatitis B (HepB) vaccine (Hib-Hep B).10 In the future, combination vaccines might include increasing numbers of components in different arrays to protect against these and other diseases, including hepatitis A, Neisseria meningitidis, Streptococcus pneumoniae and varicella (Figure 1).11
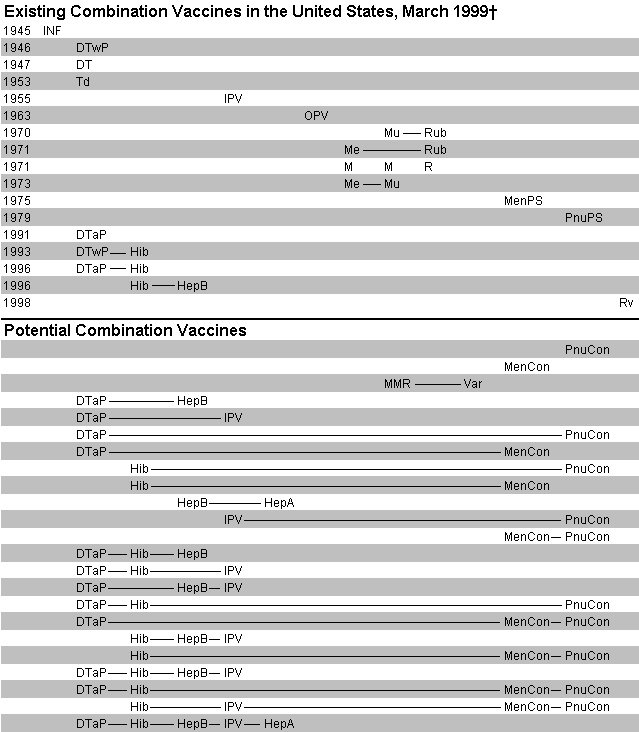
Combination vaccines have some drawbacks. Chemical incompatibility or immunologic interference when different antigens are combined into one vaccine could be difficult to overcome.12–16 Vaccine combinations that require different schedules might cause confusion and uncertainty when children are treated by multiple vaccine providers who use different products. The trend to develop combination products could encourage vaccine companies to merge to acquire the needed intellectual property.17 Competition and innovation might be reduced if companies with only a few vaccine antigens are discouraged from developing new products.
This report, published simultaneously by the Advisory Committee for Immunization Practices (ACIP),18 the American Academy of Pediatrics (AAP)19 and the American Academy of Family Physicians (AAFP),20 provides general recommendations for the optimal use of existing and anticipated parenteral combination vaccines, along with relevant background, rationale, and discussion of questions raised by the use of these products. Principal recommendations are classified by the strength and quality of evidence supporting them (Table 1).21–24
| Recommendation | Strength of evidence | Comment | |
|---|---|---|---|
| Preference for combination vaccines | B | Parent and provider surveys | |
| Manufacturer interchangeability | |||
| Permissible: diphtheria,† tetanus,† Hib, HepB | A | Good evidence | |
| Permissible: HepA | B | Preliminary data | |
| Discouraged: acellular pertussis (in DTaP, DTaP-Hib) | C | Little or no evidence | |
| Vaccine supply | C | ||
| Extra doses of vaccine antigens | |||
| Permissible: HepB, Hib, MMR, OPV, Rv, Var | A | Little or no risk of adverse events for those already immune | |
| Cautioned: tetanus† | B | Frequent revaccination could cause hypersensitivity reactions | |
Preference for Combination Vaccines
The use of licensed combination vaccines is preferred over separate injection of their equivalent component vaccines. Only combinations approved by the U.S. Food and Drug Administration (FDA) should be used (Table 2).
| DTaP: diphtheria and tetanus toxoids and acellular pertussis vaccine | ACEL-IMUNE (WLV) | |
| Certiva (NAV, distributed by ALI) | ||
| Infanrix (SBB, distributed by SB) | ||
| Tripedia (CON, distributed by PMC) | ||
| DTaP-Hib: diphtheria and tetanus toxoids and acellular pertussis and Haemophilus influenzae type b vaccine | TriHIBit* (ActHIB Hib reconstituted with Tripedia DTaP, distributed by PMC) | |
| DTwP: diphtheria and tetanus toxoids and whole-cell pertussis vaccine | Tri-Immunol (WLV)† (Generic products from other manufacturers) | |
| DTwP-Hib: diphtheria and tetanus toxoids and whole-cell pertussis and Haemophilus influenzae type b vaccine | ActHIB Hib reconstituted with DTwP (CON, distributed by PMC) | |
| TETRAMUNE (WLV) | ||
| HepA: hepatitis A vaccine | HAVRIX (SBB, distributed by SB) | |
| VAQTA (MRK) | ||
| HepB: hepatitis B vaccine | ENGERIX-B (SBB, distributed by SB) | |
| RECOMBIVAX HB (MRK) | ||
| Hib: Haemophilus influenzae type b conjugate vaccine | ||
| HbOC—oligosaccharides conjugated to diphtheria CRM197 toxin protein | HibTITER (WLV) | |
| PRP-OMP—polyribosylribitol phosphate polysaccharide conjugated to a meningococcal outer membrane protein | PedvaxHIB (MRK) | |
| PRP-T—polyribosylribitol phosphate polysaccharide conjugated to tetanus toxoid | ActHIB (PMSV, distributed by CON, PMC) | |
| OmniHIB (PMSV, distributed by SB) | ||
| PRP-D—polyribosylribitol phosphate polysaccharide conjugated to diphtheria toxoid | ProHIBiT (CON, distributed by PMC) | |
| Hip-HepB: Haemophilus influenzae type b and hepatitis B vaccine | COMVAX (Hib component = PRP-OMP) (MRK) | |
| IPV: trivalent inactivated polio vaccine (killed Salk type) | IPOL (PMSV, distributed by CON, PMC) | |
| MMR: measles-mumps-rubella vaccine | M-M-R II (MRK) | |
| OPV: trivalent oral polio vaccine (live Sabin type) | Orimune (WLV) | |
| Rv: rotavirus vaccine (live, oral, tetravalent) | RotaShield (WLV) | |
| Var: varicella (chickenpox) vaccine | VARIVAX (MRK) | |
RATIONALE
The use of combination vaccines is a practical way to overcome the constraints of multiple injections, especially for starting the immunization series for children behind schedule. The use of combination vaccines might improve timely vaccination coverage. Some immunization providers and parents object to administering more than two or three injectable vaccines during a single visit because of a child's fear of needles and pain25–30 and because of unsubstantiated concerns regarding safety.31,32
Other potential advantages of combination vaccines include (a) reducing the cost of stocking and administering separate vaccines, (b) reducing the cost for extra health care visits, and (c) facilitating the addition of new vaccines into immunization programs. The price of a new combination vaccine can sometimes exceed the total price of separate vaccines for the same diseases. However, the combination vaccine might represent a better economic value if one considers the direct and indirect costs of extra injections, delayed or missed vaccinations, and additional handling and storage.11
COMBINING SEPARATE VACCINES WITHOUT FDA APPROVAL
Immunization providers should not combine separate vaccines into the same syringe to administer together unless such mixing is indicated for the patient's age on the respective product label inserts approved by the FDA. The safety, immunogenicity and efficacy of such unlicensed combinations are unknown.33
Interchangeability of Vaccine Products
In general, vaccines from different manufacturers that protect against the same disease may be administered interchangeably in sequential doses in the immunization series for an individual patient (e.g., hepatitis A [HepA], HepB and Hib). However, until data supporting interchangeability of acellular pertussis vaccines (e.g., DTaP) are available, vaccines from the same manufacturer should be used, whenever feasible, for at least the first three doses in the pertussis series. Immunization providers who cannot determine which DTaP vaccine was previously administered, or who do not have the same vaccine, should use any of the licensed acellular pertussis products to continue the immunization series.
INTERCHANGEABILITY OF FORMULATIONS
The FDA generally licenses a combination vaccine based on studies indicating that the product's immunogenicity (or efficacy) and safety are comparable with or equivalent to monovalent or combination products previously licensed.16,34 FDA approval also generally indicates that a combination vaccine may be used interchangeably with monovalent formulations and other combination products with similar component antigens produced by the same manufacturer to continue the vaccination series. For example, DTaP, DTaP-Hib and future DTaP-combination vaccines (Figure 1) that contain similar acellular pertussis antigens from the same manufacturer may be used interchangeably, if approved for the patient's age.
INTERCHANGEABILITY OF VACCINES FROM DIFFERENT MANUFACTURERS
The licensure of a vaccine does not necessarily indicate that interchangeability with products of other manufacturers has been demonstrated. Such data are ascertained and interpreted more easily for diseases with known correlates of protective immunity (e.g., specific antibodies). For diseases without such surrogate laboratory markers, field efficacy (phase III) trials or postlicensure surveillance generally are required to determine protection.35,36
Diseases With Serologic Correlates of Immunity. Studies of serologic responses that have been correlated with protection against specific diseases support the interchangeability of vaccines from different manufacturers for HepA, HepB and Hib.
Preliminary data indicate that the two hepatitis A vaccine products currently licensed in the United States37 may be used interchangeably38 (Merck & Co., Inc., unpublished data, 1998). Hepatitis B vaccine products (i.e., HepB and Hib-HepB if age appropriate) also may be interchanged for any doses in the hepatitis B series.39
Based on subsequent data,40–42 the guidelines for Haemophilus influenzae type b disease7,43 were updated in the 1998 Recommended Childhood Immunization Schedule44–47 to indicate that different Hib vaccine products from several manufacturers may be used interchangeably for sequential doses of the vaccination series. A PRP-OMP Hib (Hib vaccine with a polyribosylribitol phosphate polysaccharide conjugated to a meningococcal outer membrane protein) or a PRP-OMP Hib-HepB vaccine might be administered in a series with HbOC Hib (Hib vaccine with oligosaccharides conjugated to diphtheria CRM197 toxin protein) or with PRP-T Hib (polyribosylribitol phosphate polysaccharide conjugated to tetanus toxoid). In such cases, the recommended number of doses to complete the series is determined by the HbOC or PRP-T product, not by the PRP-OMP vaccine.1,2 For example, if PRP-OMP Hib is administered for the first dose at age two months and another product is administered at age four months, a third dose of any of the licensed Hib vaccines is recommended at age six months to complete the primary series.
Diseases Without Serologic Correlates of Immunity. Despite extensive research, no serologic correlates of immunity have been identified for pertussis. Limited data exist concerning the safety, immunogenicity or efficacy of administering acellular pertussis vaccines (e.g., DTaP or DTaP-Hib) from different manufacturers between the fourth (at age 15 to 18 months) and fifth (at age four to six years) doses in the vaccination series.48 No data are available regarding the interchangeability of acellular pertussis products from different manufacturers for the first three pertussis doses scheduled at ages two, four and six months. Thus, use of the same manufacturer's acellular pertussis vaccine product(s) is preferred for at least the first three doses in the series.5,49
Vaccine Supply
Immunization clinics and providers should maintain a supply of vaccines that will protect children from all diseases specified in the current Recommended Childhood Immunization Schedule.1,2 This responsibility can be fulfilled by stocking several combination and monovalent vaccine products. However, not stocking all available combination and monovalent vaccines or multiple products of each is acceptable.
New and potential combination vaccines can contain different but overlapping groups of antigens (Figure 1). Thus, not all such vaccines would need to be available for the age-appropriate vaccination of children. Those responsible for childhood vaccination can stock several vaccine types and products, or they may continue to stock a limited number, as long as they prevent all diseases recommended in the immunization schedule.1,2 Potential advantages of stocking a limited number of vaccines include reducing (a) confusion and potential errors when staff must handle redundant products and formulations, (b) wastage when less commonly used products expire, (c) cold storage capacity requirements and (d) administrative overhead in accounting, purchasing and handling.
Extra Doses of Vaccine Antigens
Using combination vaccines containing some antigens not indicated at the time of administration to a patient might be justified when (a) products that contain only the needed antigens are not readily available or would result in extra injections and (b) potential benefits to the child outweigh the risk of adverse events associated with the extra antigen(s). An extra dose of many live-virus vaccines and Hib or HepB vaccines has not been found to be harmful. However, the risk of adverse reactions might increase when extra doses are administered earlier than the recommended interval for certain vaccines (e.g., tetanus toxoid vaccines and pneumococcal polysaccharide vaccine).23,50
GENERAL IMMUNIZATION PRACTICE
Patients commonly receive extra doses of vaccines or vaccine antigens for diseases to which they are immune. For example, some children receiving recommended second or third doses of many vaccines in the routine immunization series will already have immunologic protection from previous dose(s). Because serologic testing for markers of immunity is usually impractical and costly, multiple doses for all children are justified for both clinical and public health reasons to decrease the number of susceptible persons, which ensures high overall rates of protection in the population.
Extra vaccine doses also are sometimes administered when an immunization provider is unaware that the child is already up-to-date for some or all of the antigens in a vaccine (see Improving Immunization Records). During National Immunization Days and similar mass campaigns, millions of children in countries around the world are administered polio vaccine51,52 and/or measles vaccine,53,54 regardless of prior vaccination status.
EXTRA DOSES OF COMBINATION VACCINE ANTIGENS
ACIP, AAP and AAFP recommend that combination vaccines may be used whenever any components of the combination are indicated and its other components are not contraindicated.1,2 An immunization provider might not have vaccines available that contain only those antigens indicated by a child's immunization history. Alternatively, the indicated vaccines might be available, but the provider nevertheless might prefer to use a combination vaccine to reduce the required number of injections. In such cases, the benefits and risks of administering the combination vaccine with an unneeded antigen should be compared.
Live-Virus Vaccines. Administering an extra dose of live, attenuated virus vaccines to immunocompetent persons who already have vaccine-induced or natural immunity has not been demonstrated to increase the risk of adverse events. Examples of these include MMR, varicella, rotavirus and oral polio vaccines.
Inactivated Vaccines. When inactivated (killed) or subunit vaccines (which are often adsorbed to aluminum-salt adjuvants) are administered, the reactogenicity of the vaccine must be considered in balancing the benefits and risks of extra doses. Because clinical experience suggests low reactogenicity, an extra dose of Hib or HepB vaccine may be administered as part of a combination vaccine to complete a vaccination series for another component of the combination. Administration of extra doses of tetanus toxoid-containing vaccines earlier than the recommended intervals can increase the risk of hypersensitivity reactions.55–61 Examples of such vaccines include DTaP, DTaP-Hib, diphtheria and tetanus toxoids for children (DT), tetanus and diphtheria toxoids for adolescents and adults (Td), and tetanus toxoid (TT). Extra doses of tetanus toxoid-containing vaccines might be appropriate in certain circumstances, including for children who received prior DT vaccine and need protection from pertussis (in DTaP) or for immigrants with uncertain immunization histories.
IMPACT OF REIMBURSEMENT POLICIES
Administering extra antigens contained in a combination vaccine, when justified as previously described, is acceptable practice and should be reimbursed on the patient's behalf by indemnity health insurance and managed-care systems. Otherwise, high levels of timely vaccination coverage might be discouraged.
CONJUGATE VACCINE CARRIER PROTEINS
Some carrier proteins in existing conjugated Hib vaccines62 also are used as conjugates in new vaccines in development (e.g., for pneumococcal and meningococcal disease).63 Protein conjugates used in Hib conjugate vaccines include a mutant diphtheria toxin (in HbOC), an outer membrane protein from Neisseria meningitidis (in PRP-OMP), and tetanus and diphtheria toxoids (in PRP-T and PRP-D [polyribosylribitol phosphate polysaccharide conjugated to a diphtheria toxoid], respectively). Administering large amounts of tetanus toxoid carrier protein simultaneously with PRP-T conjugate vaccine has been associated with a reduction in the response to PRP64 (see “Future Research and Priorities”).
Improving Immunization Records
Improving the convenience and accuracy of transferring vaccine-identifying information into medical records and immunization registries should be a priority for immunization programs. Priority also should be given to ensuring that providers have timely access to the immunization histories of their patients.
As new combination vaccines with longer generic names and novel trade names are licensed (Figure 1), problems with accurate recordkeeping in medical charts and immunization registries will likely be exacerbated.
MONITORING VACCINE SAFETY, COVERAGE AND EFFICACY
All health care providers are mandated by law to document in each patient's medical record the identity, manufacturer, date of administration and lot number of certain specified vaccines, including most vaccines recommended for children.65,66 Although such data are essential for surveillance and studies of vaccine safety, efficacy and coverage, these records are often incomplete and inaccurate. Two major active67 and passive68,69 surveillance systems monitoring vaccine safety in the United States have detected substantial rates of missing and erroneous data (10 percent or more) in the recording of vaccine type, brand or lot number in the medical records of vaccine recipients (CDC, unpublished data, 1997). Similar rates of incomplete and incorrect vaccination medical records were encountered by the National Immunization Survey and the National Health Interview Survey (CDC, unpublished data, 1997).
PATIENT MIGRATION AMONG IMMUNIZATION PROVIDERS
Changing immunization providers during the course of a child's vaccination series is common in the United States. The 1994 National Health Interview Survey documented that approximately 25 percent of children were vaccinated by more than one provider during the first two years of life (CDC, unpublished data, 1997). Eligibility for Medicaid and resulting enrollment in Medic-aid managed-care health plans tend to be sporadic, with an average duration of nine months and a median of less than 12 months in 1993 (Health Care Financing Administration, unpublished data, 1998).
The vaccination records of children who have changed immunization providers are often unavailable and incomplete. Missing or inaccurate information regarding the vaccines received previously might preclude accurate determination of which vaccines are indicated at the time of a visit, resulting in the administration of extra doses.
STRATEGIES FOR ACCURATE VACCINE IDENTIFICATION
Potential strategies to improve the accuracy and timely availability of vaccination information include the following:
Designing and adopting a recommended, nationally standardized, uniform vaccination medical record form. A copy provided to parents could serve as a record of vaccination history for subsequent immunization providers and satisfy school entry requirements. Immunization registries could generate printouts to document vaccinations received from multiple providers and to replace misplaced forms.
Expanding and coordinating immunization registries, which track vaccinations received by children and make the information available in a convenient and timely manner to parents and authorized immunization providers with a need to know, while protecting confidentiality and privacy.
Developing technologies, standards and guidelines to improve the accuracy and convenience of recording and transferring information from the vaccine package or vial into a patient's medical record, compatible with both manual and computerized medical record systems. These methods could include standardized, peel-off identification stickers on vaccine packaging and standardized coding of vaccine identity, expiration date and lot number. Machine-readable bar codes following Uniform Code Council standards70 on vaccine packaging and/or stickers could facilitate accurate electronic transfer of this information into computerized medical record systems and immunization registries.
Future Research and Priorities
Further efforts are needed to study and obtain more data on the following key subjects related to combination vaccines:
The interchangeability of vaccines produced by different manufacturers to prevent the same disease, particularly those that differ in the nature or quantity of one or more component antigens.
The safety and efficacy of administering combination vaccines to patients who might already be fully immunized for one or more of the components.
Economic and operations research on (a) the frequency of delayed or missed vaccinations because of objections to multiple injections; (b) the costs of any increased disease burden caused by such missed vaccinations; (c) the costs of extra visits needed to comply with the routine immunization schedule; and (d) the administrative overhead and cost of errors and confusion that might result when handling a greater number of products.
The effects on immunogenicity and safety of simultaneous or repeated exposures to the same proteins used as antigens (e.g., tetanus and diphtheria toxoids) and/or as carrier components in existing and future conjugated vaccines.
Research to develop and evaluate alternative means of antigen delivery by the mucosal,71,72 parenteral73 and cutaneous routes,74–77 which would allow new and existing vaccines to be administered less painfully and more safely than with needles and syringes.78–80
| DT | Diphtheria and tetanus toxoids vaccine (for children) | |
| DTaP | Diphtheria and tetanus toxoids and acellular pertussis vaccine | |
| DTaP-Hib | Diphtheria and tetanus toxoids and acellular pertussis and Haemophilus influenzae type b vaccine | |
| DTP | Diphtheria and tetanus toxoids and pertussis vaccine (unspecified pertussis antigens) | |
| DTP-Hib | Diphtheria and tetanus toxoids and pertussis and Haemophilus influenzae type b vaccine (unspecified pertussis antigens) | |
| DTwP | Diphtheria and tetanus toxoids and whole-cell pertussis vaccine | |
| DTwP-Hib | Diphtheria and tetanus toxoids and whole-cell pertussis and Haemophilus influenzae type b vaccine | |
| HepA | Hepatitis A vaccine | |
| HepB | Hepatitis B vaccine | |
| Hib | Haemophilus influenzae type b conjugate vaccine | |
| PRP-OMP Hib | Polyribosylribitol phosphate polysaccharide conjugated to a meningococcal outer membrane protein | |
| PRP-T Hib | Polyribosylribitol phosphate polysaccharide conjugated to tetanus toxoid | |
| HbOC Hib | Oligosaccharides conjugated to diphtheria CRM197 toxin protein | |
| PRP-D Hib | Polyribosylribitol phosphate polysaccharide conjugated to diphtheria toxoid | |
| Hib-HepB | Haemophilus influenzae type b and hepatitis B vaccine | |
| Hib-HepB-IPV | Haemophilus influenzae type b, hepatitis B and trivalent inactivated polio vaccine | |
| INF | Influenza vaccine | |
| IPV | Trivalent inactivated polio vaccine (killed Salk type) | |
| Me | Measles vaccine | |
| Me-Rub | Measles and rubella vaccine | |
| MenCon | Meningococcal (Neisseria meningitidis) conjugate vaccine | |
| MenPS | Meningococcal (Neisseria meningitidis) polysaccharide vaccine | |
| MMR | Measles-mumps-rubella vaccine | |
| MMR-Var | Measles-mumps-rubella and varicella vaccine | |
| Mu | Mumps vaccine | |
| Mu-Rub | Mumps and rubella vaccine | |
| OPV | Trivalent oral polio vaccine (live Sabin type) | |
| PnuCon | Pneumococcal (Streptococcus pneumoniae) conjugate vaccine | |
| PnuPS | Pneumococcal (Streptococcus pneumoniae) polysaccharide vaccine | |
| Rub | Rubella vaccine | |
| Rv | Rotavirus vaccine | |
| Td | Tetanus and diphtheria toxoids vaccine (for adolescents and adults) | |
| TT | Tetanus toxoid vaccine | |
| Var | Varicella (chickenpox) vaccine | |