
This is a corrected version of the article that appeared in print.
Am Fam Physician. 1999;60(6):1743-
The etiologies of respiratory failure, shock, cardiopulmonary arrest and dysrhythmias in children differ from those in adults. In 1988, the American Heart Association implemented the pediatric advanced life support (PALS) program. Major revisions to the program were made in 1994, with further revisions in 1997. The PALS program teaches a systematic, organized approach for the evaluation and management of acutely ill or injured children. Early identification and treatment of respiratory failure and shock in children improve survival, from a dismal 10 percent to an encouraging 85 percent. Family physicians who care for acutely ill or injured children have a tremendous opportunity to save lives through implementation of the PALS information.
In 1983, the American Heart Association (AHA) recommended the development of a course in pediatric advanced life support (PALS) as a means of fulfilling the need for resuscitation guidelines and training specifically for children. The first edition of the PALS manual was published in 1988, and the first PALS courses began that year.1 The PALS program underwent major revisions in 1994 following recommendations from the 1992 National Conference on Cardiopulmonary Resuscitation and Emergency Cardiac Care. The most recent revisions in the PALS provider and instructor manuals were completed in 1997. This article summarizes information from the PALS program but does not serve as a replacement for completing a PALS course.
Prevention of Cardiopulmonary Arrest
The etiology of adult cardiac arrest is typically a sudden dysrhythmia, most commonly ventricular fibrillation. Pediatric cardiopulmonary arrest results when respiratory failure or shock is not identified and treated in the early stages. Respiratory infection, bronchospasm, foreign body aspiration, drowning, trauma, vomiting or diarrhea, sepsis, supra-ventricular tachycardia and, rarely, underlying cardiac abnormalities can produce respiratory failure and shock in children (Table 1). Early recognition and intervention prevents deterioration to cardiopulmonary arrest and probable death.2
| Bronchospasm |
| Burns |
| Congenital cardiac abnormalities |
| Drowning |
| Dysrhythmias |
| Foreign body aspiration |
| Gastroenteritis |
| Seizures |
| Sepsis |
| Trauma |
| Upper and lower respiratory tract infection |
Approximately 10 percent of children who progress to cardiopulmonary arrest are successfully resuscitated.1,3 However, children who are resuscitated when only respiratory arrest is present have a 75 to 93 percent survival rate.4–6 In one study, 92 percent of such children had no neurologic impairment.5 Pediatric advanced life support begins with early recognition and management of respiratory failure and shock. Because the adult and pediatric etiologies of cardiopulmonary arrest differ, a different approach to assessment and intervention is required in the pediatric population.
Cardiopulmonary Assessment
The ABCs (airway, breathing, circulation) are used in the PALS program to develop an organized approach for pediatric advanced life support. A 30-second rapid cardiopulmonary assessment is structured around the ABCs.
AIRWAY ASSESSMENT
Airway (A) assessment is performed first to determine the child's ability to ventilate. The airway is identified as clear, maintainable with repositioning or unmaintainable without intubation or foreign body removal.1 The airway must be clear and patent for successful ventilation.
BREATHING ASSESSMENT
After the airway assessment, breathing (B) is assessed to determine the child's ability to oxygenate. Respiratory rate, respiratory effort, breath sounds (air entry) and skin color reflect oxygenation and provide objective measurements of breathing (Table 2). A respiratory rate of less than 10 or greater than 60 is an ominous sign of impending respiratory failure. Use of accessory muscles, manifested by supraclavicular, intercostal, subcostal or sternal retractions, as well as the presence of grunting or nasal flaring, are signs of increased work of breathing. Auscultation of breath sounds provides a clinical determination of tidal volume. Skin color deteriorates from pink, to pale, to mottled, to blue as hypoxemia progresses.1
| Age | Respiratory rate per minute |
|---|---|
| <1 year | 24 to 38 |
| 1 to 3 years | 22 to 30 |
| 4 to 6 years | 20 to 24 |
| 7 to 9 years | 18 to 24 |
| 10 to 14 years | 16 to 22 |
| 15 to 18 years | 14 to 20 |
CIRCULATION ASSESSMENT
The third assessment is circulation (C), which reflects perfusion. Shock is a physiologic state where delivery of oxygen and substrates (perfusion) is inadequate to meet tissue metabolic demands. Adequacy of perfusion is quantified through heart rate, pulse quality, level of consciousness, capillary refill, extremity temperature, skin color, urine output and blood pressure.1
Heart rate is the most sensitive parameter for determining perfusion and oxygenation in children (Table 3). A rapid heart rate (over 140 beats per minute) necessitates clinical evaluation to rule out a pathologic etiology. A heart rate of less than 60 beats per minute provides inadequate cardiac output and is an ominous sign. Chest compressions should be instituted until therapeutic interventions increase the heart rate to more than 60 beats per minute.1,4
Pulse quality reflects cardiac output. Peripheral perfusion can be assessed by comparing pulse quality and skin temperature at a proximal site with that at a distal site. Capillary refill provides another measurement of peripheral perfusion. In warm ambient air with the extremity slightly above the level of the heart, normal capillary refill on the palm or sole is less than three seconds.1,4
The brain and the kidneys are vital organs. Brain perfusion determines the level of consciousness. The level of consciousness can be quantitated by using the mnemonic AVPU: alert, responds to verbal stimuli, responds to painful stimuli and unresponsive. Urine output can be monitored during resuscitation to determine kidney perfusion. Urine output of 1 to 2 mL per kg per hour reflects adequate renal perfusion.1
Blood pressure is an adjunct measurement that reflects the patient's ability to compensate when in shock. Minimal acceptable blood pressures (fifth percentile) vary with the child's age (Table 4). Hypotension (a blood pressure below the fifth percentile) indicates decompensated shock; 25 percent of the blood volume must be lost before a drop in blood pressure occurs.4 Thus, hypotension occurs late in shock. If shock is identified early, before hypotension (decompensation), treatment can be instituted so irreversible shock and death are averted.
| Age | Minimal acceptable blood pressure (fifth percentile) |
|---|---|
| Birth to 1 month | 60 mm Hg |
| >1 month to 1 year | 70 mm Hg |
| >1 year | 70 mm Hg + (2 × age in years) |
Determination of Physiologic Status
Rapid cardiopulmonary assessment permits determination of the child's physiologic status: respiratory failure, shock or cardiopulmonary failure. Respiratory failure is defined as inadequate ventilation or oxygenation, or both, that leads to an elevated partial pressure of arterial carbon dioxide (PaCO2) and/or a decreased partial pressure of arterial oxygen (PaO2), resulting in acidosis. Potential and probable respiratory failure can be identified clinically, permitting interventions without the delay of a blood gas determination. Potential respiratory failure is present if the clinical assessment reveals inadequate ventilation or oxygenation—that is, any abnormality in the airway or breathing portion of the cardiopulmonary assessment. Lack of response to airway maneuvers and oxygen, or any deterioration, is considered probable respiratory failure. Probable respiratory failure requires more aggressive intervention, such as bag-valve-mask ventilation or intubation.1,4
Management Priorities
Once the child's physiologic status is determined, management specific to the physiologic status is initiated using the ABC format. All children receive oxygen in the highest concentration available, cardiac monitoring and pulse oximetry, if available. Pulse oximetry is an inaccurate measure of oxygen saturation when peripheral perfusion is impaired.
RESPIRATORY FAILURE
Children with potential or probable respiratory failure should receive rapid, aggressive airway management. The highest concentration of oxygen available is delivered. The child is maintained in a position of comfort. Basic airway maneuvers are employed to open and maintain the airway: oral or nasopharyngeal airway placement; neutral, in-line positioning of the head, neck and shoulders; and anterior displacement of the chin or jaw to facilitate an open mouth. Foreign bodies are removed, if present.
Bag-valve-mask ventilation is initiated when these measures are inadequate or if bradypnea or apnea develops. Intubation is performed if prolonged ventilation is required; if the child does not adequately respond to bag-valve-mask ventilation; or if the airway requires protection from emesis. Matching the endotracheal tube to the size of the nares or fifth finger provides an adequate estimate of the appropriate tube size. Charts and length-based tapes are available for more accurate determination of endotracheal tube size.
SHOCK
If the child is assessed to be in shock, oxygen administration and monitoring are followed by initiation of vascular access. Crystalloid (normal saline or lactated Ringer's) solutions are used for rapid (delivered in less than 20 minutes) fluid boluses of 20 mL per kg until the shock is resolved. The peripheral proximal upper extremity is the location of choice for intravenous administration of crystalloid infusion. If peripheral access cannot be achieved in three attempts or 90 seconds in a child younger than six years of age, intra-osseous vascular access in the proximal tibia or distal femur should be initiated.4 If intra-osseous access is unsuccessful or if the child is over six years of age, central venous access (preferably femoral) should be obtained. Urine output should be monitored to determine vital organ perfusion in response to resuscitation.1,4
Hypovolemic shock requires repeated fluid boluses until the child's condition is stable and perfusion parameters have normalized. Shock secondary to traumatic blood loss may require blood replacement if hypotension (decompensated shock) occurs or perfusion parameters have not normalized after a total of 40 to 60 mL per kg of crystalloid solution have been administered. Children in septic shock and cardiogenic shock should initially receive crystalloid solution (boluses of 20 mL per kg). Inotropic agents should be considered if septic or cardiogenic shock persists after intravenous volume has been repleted (repletion typically requires a total of 40 to 60 mL per kg of crystalloid solution).1 Epinephrine is usually the inotrope of choice (Table 5).
| Agent | Intravenous dosage | Indications |
|---|---|---|
| Epinephrine | 0.1 to 1.0 μg per kg per minute (continuous infusion) | Symptomatic bradycardia |
| Shock (cardiogenic, septic, anaphylactic) | ||
| Hypotension | ||
| Dopamine | 2 to 5 μg per kg per minute (continuous infusion) 10 to 20 μg per kg per minute (continuous infusion) | Low doses: improve renal and splanchnic blood flow High doses: useful in the treatment of hypotension and shock in the presence of adequate intravascular volume |
| Dobutamine | 2 to 20 μg per kg per minute (continuous infusion) | Normotensive cardiogenic shock |
CARDIOPULMONARY FAILURE
Children in cardiopulmonary failure have global deficits in airway, breathing and circulation. Oxygen is delivered in the highest concentration available, usually by bag-valve-mask ventilation. A cardiac monitor is applied. Vascular access is obtained.
The child's response to ventilation and oxygenation guides further interventions. If the child improves with ventilation and oxygenation, the inciting etiology was likely respiratory failure. Definitive respiratory interventions such as intubation and foreign body removal are completed. However, if ventilation and oxygenation are maximized and signs of shock persist, crystalloid replacement is initiated with boluses of 20 mL per kg infused over less than 20 minutes. Inotropic agents are added if indicated.1
GLUCOSE LEVEL
Serum glucose concentration should be determined in all ill or injured children, particularly those who are undergoing resuscitation. Glucose replacement is provided with a maximum concentration of 25 percent dextrose in water, 2 to 4 mL per kg (0.5 to 1 g per kg) intravenously over 20 to 30 minutes for hypoglycemia. In neonates, 10 percent dextrose in water (0.5 to 1 g per kg) is recommended for glucose replacement.4
Dysrhythmias
Dysrhythmias are uncommon in the pediatric population. They typically occur secondary to hypoxemia or shock rather than being the cause of respiratory failure, shock or cardiopulmonary failure. Supraventricular tachycardia occurs as a primary dysrhythmia, particularly in the first year of life.
BRADYCARDIA
Bradycardia is the most common dysrhythmia in the pediatric population.4,7,8 Since the etiology of bradycardia is usually hypoxemia, initial management is ventilation and oxygenation while perfusion is maintained with chest compressions in children with a heart rate of less than 60 beats per minute. If these measures do not restore the heart rate, epinephrine is administered to treat the bradycardia. Intravenous or intraosseous epinephrine is given in a dose of 0.01 mg per kg of the 1:10,000 concentration of epinephrine, which is 0.1 mL per kg in volume. Endotracheal tube administration of epinephrine is given as a dose of 0.1 mg per kg of the 1:1,000 concentration of epinephrine, which is 0.1 mL per kg in volume (Table 6). This dose may be repeated every three to five minutes.
| Dysrhythmia | Route of administration | Dosage and concentration |
|---|---|---|
| Bradycardia | IV, IO | 0.01 mg per kg (0.1 mL per kg), 1:10,000 |
| ET | 0.1 mg per kg (0.1 mL per kg), 1:1,000 | |
| [ corrected] Asystole, ventricular fibrillation, pulseless electrical activity | Initial IV, IO | 0.01 mg per kg (0.1 mL per kg), 1:10,000 |
| Subsequent IV, IO | 0.1 mg per kg (0.1 mL per kg), 1:1,000 | |
| All ET | 0.1 mg per kg (0.1 mL per kg), 1:1,000 |
ASYSTOLE
Epinephrine remains the drug of choice for asystole in children. Atropine is not indicated. The initial doses are identical to those for bradycardia (Table 6). However, subsequent doses, which are administered every three to five minutes, are identical for all routes (i.e., intravenous, intraosseous and endotracheal): 0.1 mg per kg, given as 0.1 mL per kg of the 1:1,000 concentration of epinephrine.1,4 Although the benefit of this higher dose of epinephrine has been questioned,8 this is the dose currently recommended by the AHA.
SUPRAVENTRICULAR TACHYCARDIA
Supraventricular tachycardia—a heart rate greater than 220 in infants and greater than 180 in children—is the most common dysrhythmia in the first year of life. Management of the child whose condition is stable, with no signs of respiratory compromise or shock and a normal blood pressure, includes administration of high-flow oxygen, cardiac monitoring and pediatric cardiology consultation (Figure 1). If proximal intravenous access is easily obtainable, adenosine in a dose of 0.1 mg per kg (initial maximum dose: 6 mg) may be given by rapid intravenous push.4 The dose of adenosine may be doubled (maximum single dose: 12 mg) and repeated if supraventricular tachycardia is not converted to sinus rhythm with the initial dose.
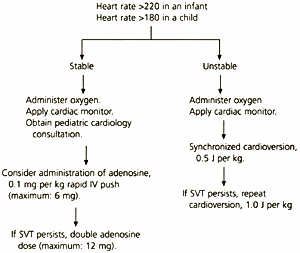
Although verapamil (Calan) may be used in adults with supraventricular tachycardia, it is contraindicated in children under the following circumstances: age under one year; in the presence of congestive heart failure or myocardial depression; in children receiving beta-adrenergic blockers; and in the presence of a possible bypass tract (i.e., Wolff-Parkinson-White syndrome).4
VENTRICULAR TACHYCARDIA
Ventricular tachycardia is typically secondary to structural cardiac disease, prolonged QT syndrome, hypoxemia, acidosis, electrolyte imbalance, drug toxicity or poisoning. Children with signs of shock who have palpable pulses but whose condition is unstable receive synchronized cardioversion beginning at 0.5 J per kg (Figure 2). A lidocaine (Xylocaine) bolus (1 mg per kg) is also administered, followed by continuous infusion at a rate of 20 to 50 μg per kg per minute. The etiology of ventricular tachycardia must be identified and managed.1,4
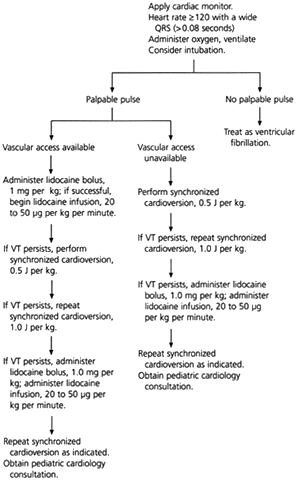
PULSELESS ELECTRICAL ACTIVITY
Pulseless electrical activity is uncommon in children. Pulseless electrical activity is secondary to one of the following underlying etiologies: hypoxemia, hypovolemia, hypothermia, hypoglycemia, hyperkalemia, cardiac tamponade, tension pneumothorax, severe acidosis or drug overdose. Successful resuscitation depends on identification and treatment of the underlying etiology. Epinephrine, administered in the same dose that is used to treat asystole, may be used until the etiology is fully addressed.1,4
VENTRICULAR FIBRILLATION AND PULSELESS VENTRICULAR TACHYCARDIA
Ventricular fibrillation and pulseless ventricular tachycardia are highly uncommon dysrhythmias in children. The treatment of these dysrhythmias consists of undelayed, successive defibrillation, beginning at 2 J per kg, then doubling to 4 J per kg for a maximum of three consecutive defibrillations or until conversion to sinus rhythm occurs (Figure 3). If defibrillation is unsuccessful, cardiopulmonary resuscitation, intubation and vascular access are performed. Epinephrine and lidocaine are alternated with defibrillation until successful conversion to sinus rhythm.1,4

Postresuscitation Care
Once a child is resuscitated, medical care and reassessment must be ongoing. Laboratory and radiologic information is obtained. The etiology of respiratory failure or shock is determined. The level of care required for the child is ascertained. If the child requires transfer to another hospital, the method of transfer is facilitated.1,4