
Am Fam Physician. 1999;60(7):1969-1980
The diagnosis of venous thromboembolic disease, and pulmonary embolism in particular, remains problematic. Physicians should strongly consider empiric anticoagulation if the best available diagnostic tests are inconclusive, because treatment is usually safe and successful. Twice-daily subcutaneous low-molecular-weight heparin, dosed without monitoring, may eventually replace standard heparin for most treatment of venous thromboembolism, but it is not yet labeled for the treatment of pulmonary embolism. Deep venous thrombosis and pulmonary embolism should be treated with anticoagulants rather than inferior vena cava filters, even in oncology patients, unless anticoagulation is contraindicated; if so, when the contraindication remits, anticoagulation should be employed. The most effective prophylaxis of venous thromboembolism in at-risk patients should be used, with prolonged duration if evidence from clinical trials supports efficacy and safety. Low-dose warfarin should be used to prevent venous thrombosis and indwelling central venous catheter thrombosis in patients with cancer.
Few common medical conditions are as difficult to diagnose as pulmonary embolism. Treatment is usually satisfactory, but optimal treatment is controversial. An estimated 300,000 Americans suffer pulmonary embolism each year. Among those in whom the condition is diagnosed, 2 percent die within the first day and 10 percent have recurrent pulmonary embolism; the death rate among the latter group is 45 percent.1 Thus, suspicion of pulmonary embolism and its prevention are critically important, even while consensus on diagnosis and optimal treatment is not at hand.
Compared with pulmonary embolism, deep venous thrombosis (DVT) can be less difficult to diagnose, and alone it only very rarely causes death (e.g., from complications of phlegmasia in very ill patients). However, its diagnosis, treatment and prevention are all controversial.
Diagnosis
Two recent studies conducted in different institutions highlight the difficulty in diagnosing pulmonary embolism when the diagnosis could make a difference.4,5 Table 1 shows remarkably similar results from these two hospitals, in which 4 to 5 percent of autopsied patients had pulmonary embolism as the cause of death, rather than merely in association with death.
| Hospital | Number of deaths (%) | ||
|---|---|---|---|
| Pulmonary embolism caused death | Pulmonary embolism unsuspected before autopsy | ||
| St. Michael's Hospital, University of Toronto, Ontario (48 months) | 44 (4) | 30 (68) | |
| Henry Ford Hospital, Detroit: PIOPED site (21 months) | 20 (5) | 14 (70) | |
In both series,4,5 the charts of 70 percent of patients dying of pulmonary embolism recorded no suspicion of the diagnosis. In one of the hospitals,5 the fatal pulmonary emboli were missed despite simultaneous and ongoing intense recruitment and evaluation of patients with suspected pulmonary embolism for entry into a multicenter clinical study (the Prospective Investigation of Pulmonary Embolism Diagnosis [PIOPED] study).1
These results argue for defending physicians who, despite conscientious medical care, miss fatal pulmonary emboli in their patients. The results also argue for highly aggressive prophylaxis if it can be given safely.
LUNG SCANS
Ventilation-perfusion lung scans are quite helpful when they are normal or high probability, but controversy exists concerning the value of other results. Nuclear medicine physicians customarily discuss results in several categories: high probability, intermediate probability, low probability, nearly normal and normal.
The PIOPED study6 suggested that combining clinical suspicion (high, intermediate or low) with the lung scan result is very helpful in making or excluding the diagnosis of pulmonary embolism. Although theoretically attractive, it is doubtful that the data warrant such firm interpretation for several reasons. First, clinical probability was not explicitly scored; rather, it was a “gestalt” opinion of a study investigator. In the PIOPED study, clinical probability was scored as zero to 19 percent, 20 to 79 percent and 80 to 100 percent.
Absent explicit scoring criteria, it is likely that one physician's scoring of a patient would differ from another physician's scoring. For example, one physician might score a patient's presentation as an 18 percent likelihood for pulmonary embolism, whereas another physician might score it as a 22 percent likelihood. Deriving this patient's overall probability for pulmonary embolism by PIOPED criteria using the lung scan result would differ markedly, depending on whether the 18 or 22 percent likelihood was used. Hence, PIOPED's clinical probability scoring basis, its generalizability outside the expert study investigators and its reproducibility among the same physicians or with similar patient presentations are unknown. Other authors have designed explicit decision rules to score clinical probabilities in such patients.7
My calculated 95 percent confidence intervals for the probabilities reported in the PIOPED study6 are presented in Table 2. Dilemmas become apparent with close analysis. For example, in the patient whose scan is read as “low probability” and in whom the clinician felt there was a “high clinical probability” (80 to 100 percent) of pulmonary embolism, the PIOPED study reported that only 40 percent of such patients will have pulmonary embolism proved by angiography. However, because of the limited numbers of patients in this category (and many other categories within PIOPED), the 95 percent confidence interval for 40 percent includes values from 16 to 68 percent. If the lung scan results are used alone, however, PIOPED showed that a high probability scan will be confirmed by pulmonary arteriography 88 percent of the time.
| Scan result | Clinical probability, percent (95 % CI *) | ||
|---|---|---|---|
| 80 to 100 | 20 to 79 | 0 to 19 | |
| High | 96 (82 to 99) | 88 (78 to 94) | 56 (21 to 86) |
| Intermediate | 66 (49 to 80) | 28 (22 to 34) | 16 (8 to 27) |
| Low | 40 (16 to 68) | 16 (11 to 22) | 4 (1 to 11) |
| Normal | 0 (0 to 52) | 6 (2 to 16) | 2 (0 to 9) |
Both PIOPED and other studies have shown that patients with normal lung scans have an incidence of symptomatic venous thromboembolic disease in the next several months of only 1 percent.6 Thus, these two scan categories—high probability and normal—can be used with reasonable confidence for clinical decision-making. However, so-called “intermediate” and “low probability” scans should be viewed as indeterminate, and further studies or empiric treatment (or both) should follow.
ULTRASONOGRAPHY
Ultrasound examinations of the legs often follow indeterminate lung scans. The conventional wisdom that DVT and pulmonary embolism are one disease is best forgotten in seeking to diagnose pulmonary embolism, because fewer than one half of such patients have signs or symptoms in the legs,8 and fewer than 30 percent of patients with proven pulmonary embolism had an abnormal proximal compression ultrasound examination in one recent study.9
Fortunately, among patients in whom pulmonary embolism was suspected and the lower extremity ultrasound examination was abnormal, the diagnosis was proved (high-probability lung scan or pulmonary arteriogram) in 90 percent. Like a high-probability lung scan, an abnormal ultrasound test provides sufficient confirmation in a patient in whom pulmonary embolism is truly suspected. However, among patients with suspected pulmonary embolism and a normal ultrasound test, 40 percent were still found to have the disease.9
Overall, strategies that focus on the legs to diagnose pulmonary embolism will be helpful in patients with positive ultrasound examinations—perhaps 13 percent of patients who present with suspected pulmonary embolism.9 A negative leg study does not exclude pulmonary embolism in the remaining 87 percent of patients.
In contrast, compression ultrasound examination of the proximal lower extremity veins is a highly sensitive, specific and predictive test for proximal DVT in patients with symptomatic legs. For symptomatic outpatients with suspected proximal or distal DVT, a screening ultrasound examination with a one-week follow-up test appears sufficient to help make treatment decisions.10
We now have some explanations for the inaccuracy of ultrasonography in pulmonary embolism (as well as in asymptomatic DVT,11 described below). First, outpatients with symptomatic DVT have had their symptoms an average of five days, have occlusive clots and have not received thromboprophylaxis. The first two features may not be typical of most patients with pulmonary embolism, and the third is not typical of patients with pulmonary embolism who develop the disease in the hospital. Second, leg thromboses that are presymptomatic or are formed while patients are receiving antithrombotic therapy are usually not occlusive and are softer (i.e., compressible). These clots evade both compression and Doppler-aided (duplex or color Doppler) detection. Third, pulmonary embolism from an upper extremity source is increasingly being identified.12
OTHER TECHNOLOGIES
Other emerging diagnostic technologies require evaluation and further proof of effectiveness in patient management before they supplant pulmonary arteriography. Contrast-enhanced spiral computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI) are being promoted for use in the diagnosis of pulmonary embolism.13,14 However, neither technique has sufficient correlation with pulmonary arteriography for subsegmental emboli, which occur commonly.15 Moreover, contrast-enhanced spiral CT misses central clots in the middle (right) and lingular (left) pulmonary arteries because of their nearly horizontal take-off from the hila.
A further technical problem requiring solution in institutions and possibly in individual patients is development of a protocol for optimal timing and dosage of radiocontrast material. Published studies of contrast-enhanced spiral CT and MRI have individualized these protocols depending on patient age and site of venous access for the injection of contrast material, making it questionable that performing these tests in hospitals not conducting pulmonary embolism spiral CT research will satisfactorily rule out pulmonary embolism.
Central artery defects seen in both coronal and sagittal views are usually true positive, although false positives have occurred in studies reported by expert radiologists.
SUGGESTED APPROACH
This leaves us at present with pulmonary arteriography (a safe procedure in experienced hands) for use when the lung scan is indeterminate or when discordant results and the patient's medical condition require the best proof of pulmonary embolism. For patients with symptoms of leg DVT, compression ultrasound examination will reliably detect the presence or absence of a proximal clot.
As noted previously, serial compression ultrasound examination, with two studies performed a week apart, is an alternative in patients with leg symptoms and a negative initial study. For more complicated patients, including those without leg symptoms and those with chronic leg-vein changes or questions about recurrent leg-vein thrombosis, contrast venography may still be required to make the diagnosis and determine the need for treatment.
Table 3 outlines a suggested approach to the diagnosis of pulmonary embolism.
| Test | Clinical suspicion and management | ||
|---|---|---|---|
| Lung scan | Moderate to high clinical suspicion | Low clinical suspicion (e.g., rule out pulmonary embolism) | |
| High probability | Diagnosis confirmed; stop testing | Probably pulmonary embolism | |
| Normal | Do not treat | Not pulmonary embolism | |
| Indeterminate | Treat; test further | Consider treatment pending further testing | |
| After lung scan has been obtained | Moderate to high clinical suspicion; scan indeterminate | Low clinical suspicion; scan indeterminate or high probability | |
| Compression ultrasound of proximal leg veins | |||
| Abnormal | Probably pulmonary embolism; treat | Probably pulmonary embolism; treat | |
| Normal | Test further | Test further | |
| D-dimer assay | |||
| Elevated | Consistent with pulmonary embolism | Consistent with pulmonary embolism | |
| Normal | Doubt pulmonary embolism; consider other diagnoses | Probably not pulmonary embolism | |
| Spiral/electron-beam contrast CT or contrast MRI | |||
| Abnormal | Probably pulmonary embolism; treat | Probably pulmonary embolism; treat | |
| Normal | Pulmonary embolism less likely | Pulmonary embolism less likely | |
| Pulmonary arteriogram | |||
| Abnormal | Diagnosis confirmed; treat | Diagnosis confirmed; treat | |
| Normal | Diagnosis probably excluded | Diagnosis excluded | |
Treatment
A recently published account by a physician patient relates the obstacles associated with modern hospital treatment of thromboembolism by intravenous heparin infusion.16 Unfractionated heparin given intravenously remains a standard treatment, but low-molecular-weight heparin is becoming a desirable substitute. Low-molecular-weight heparin is dosed by patient weight at a total daily dosage of over twice the prophylactic dosage.
Currently, three low-molecular-weight heparins—enoxaparin (Lovenox), dalteparin (Fragmin) and ardeparin (Normiflo)—and the heparinoid danaparoid (Orgaran) are marketed in the United States. Although there is extensive experience with some of these agents, as well as other low-molecular-weight heparins, in the treatment of thromboembolism and several have been approved for this use in other countries, the U.S. Food and Drug Administration (FDA) has as yet labeled only one of them (enoxaparin) for the treatment of venous thromboembolism.
Perhaps the largest published experience is with enoxaparin, which has been shown to be safe and effective in a dosage of 1 mg per kg given subcutaneously twice daily.17 Enoxaparin has been FDA labeled at this dosage for inpatients and outpatients and has also been labeled at a dosage of 1.5 mg per kg once daily for inpatients. Dalteparin, also available in the United States, has been used in dosages of 100 anti-Factor Xa (anti-Xa) units per kg given subcutaneously twice daily18 and 200 anti-Xa units per kg given subcutaneously once daily.19
Low-molecular-weight heparin is given without a bolus and without monitoring because of its greater than 90 percent bioavailability after subcutaneous injection. This bioavailability means that not only is the drug readily absorbed into the circulating plasma but, unlike unfractionated heparin, it undergoes minimal nonspecific protein binding. Hence, predictable levels follow injection in most patients. Moreover, this predictability prevents overly intense anticoagulation, an important risk factor for bleeding with the use of unfractionated heparin.
Further contributing to the safety of at least enoxaparin and probably other low-molecular-weight heparins is the much reduced incidence of clinically apparent heparin-induced thrombocytopenia and laboratory-demonstrable antiheparin antibodies provoked with their use. This is in contrast to the situation with the use of unfractionated heparin.20
Because the half-life of low-molecular-weight heparin is twice that of unfractionated heparin, it remains in plasma for 12 to 16 hours, allowing twice-daily injection. In patients who otherwise do not require hospitalization, home treatment of DVT with twice-daily low-molecular-weight heparin has repeatedly been proved safe and effective.17,21,22
Two frequent questions concern once-daily dosing and how to dose by weight in obese patients. One study concluded that once-daily low-molecular-weight heparin and intravenous unfractionated heparin are equivalent.23 Another study found that twice-daily dosing was probably superior to once-daily treatment.18
A recent FRAXODI trial24 omitted an unfractionated heparin comparison group and compared twice-daily and once-daily subcutaneous treatment with nadroparin, a low-molecular-weight heparin not available in the United States. The study found statistical equivalence but a trend toward superiority of once-daily treatment (recurrence and major bleeding rates of 4.1 percent and 1.3 percent, respectively, in the once-daily group, compared with recurrence and major bleeding rates of 7.2 percent and 1.2 percent, respectively, in the twice-daily group).
Another study found equivalence for unfractionated heparin and twice-daily and once-daily enoxaparin, with a trend favoring twice-daily treatment.25
With the exception of the FRAXODI trial, the studies purporting to show equivalence for once-daily low-molecular-weight heparin and the other treatments are unconvincing. One problem arises from the nature of the study design for equivalence trials. In designing such trials, investigators prospectively set a difference in event rates (e.g., recurrent thromboembolism) that they will declare to be clinically significant and choose a sample size that provides statistical significance if that difference is reached. One such study predicted a 5 percent thromboembolism recurrence rate for the control group and required the rate in the once-daily low-molecular-weight heparin group to be an additional 5 percent different to count as a clinically important difference.23
Requiring such large differences between treatments to show lack of equivalence permits the investigator to use a smaller sample size and to complete the trial more quickly. However, this approach runs the important risk of declaring inferior treatments to be “equivalent.” This is not the best way to find the safest and most effective dose.
The second problem with these studies is lack of a scientifically plausible explanation for once-daily dosing. The low-molecular-weight heparins currently available in the United States are renally eliminated and have half-lives of four to five hours after subcutaneous injection. The larger (usually approximately doubled) once-daily doses prolong plasma levels by a single half-life (about four to five hours, but not 12 hours) at the risk of reaching higher (potentially prohemorrhagic) levels initially.
Current theories of thrombosis treatment and propagation suggest that inhibiting thrombin generation is important. The minimum number of hours that inhibition of thrombin generation is needed during treatment is unknown. However, a scientific approach would suggest that if maintaining a steady-state level of inhibition (with low-molecular-weight heparin) is safely accomplished by 12-hourly injections, why should that benefit be risked to gain just one fewer subcutaneous injection daily for five to seven days? Until the FRAXODI study result is replicated in a similar study designed to discern clinically relevant differences in outcome, ideally with inclusion of a group receiving unfractionated heparin, the equivalence of once-daily dosing will remain questionable.
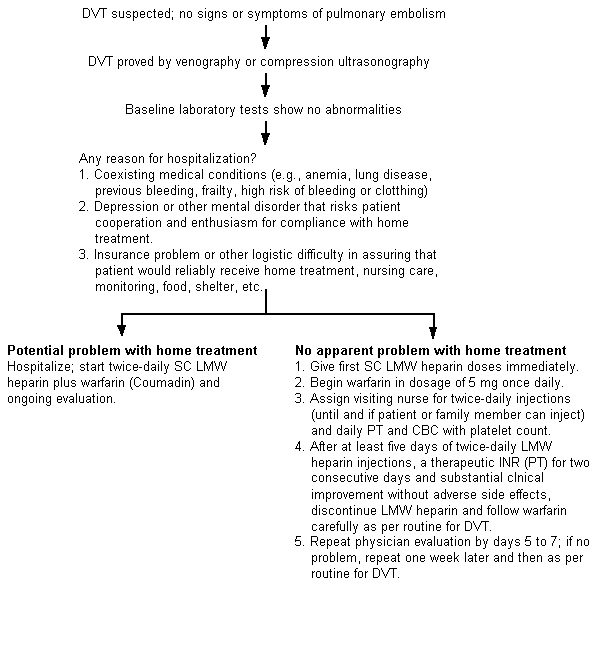
Although controlled studies have shown safe and effective treatment of DVT with low-molecular-weight heparin in outpatients and pulmonary embolism in hospitalized patients—all in selected patients—no such data support once-daily dosing out of the hospital. Figure 1 presents suggested conservative guidelines for deciding which patients with DVT to manage outside the hospital.
Little data are available on the dosing of low-molecular-weight heparin in obese patients. Clinical trials have included few patients weighing more that 110 kg (242 lb). The plasma volume of a person weighing 200 kg (440 lb) is not twice that of one weighing 100 kg (220 lb), but the volume of distribution of low-molecular-weight heparin may be twice or nearly twice as great. Like unfractionated heparin, low-molecular-weight heparin has an unusual and variable volume of distribution in normal subjects and patient volunteers.
In both extremely large and small patients and those with a creatinine clearance of less than 40 mL per minute, the dose of low-molecular-weight heparin can be estimated based on the patient's weight, but plasma anti-Xa levels should be measured after subcutaneous injection. Automated or partially automated anti-Xa level measurement kits are commercially available for use in existing coagulation laboratory devices. Although not routinely required, this relatively inexpensive assay should be available to assist in urgent care of infants, obese patients and those requiring danaparoid treatment for heparin-induced thrombocytopenia. There are no comprehensive studies to support this recommendation, but data on several low-molecular-weight heparins suggest the following targets: up to 0.8 unit per mL at one hour after a dose, 0.5 to 0.8 unit per mL at 4 hours after a dose and not lower than 0.3 unit per mL just before the next dose 12 hours later.26 In the absence of renal failure, accumulation is unlikely, and verifying the range once is sufficient unless complications occur.
Table 4 summarizes recommendations for drug treatment of acute pulmonary embolism and DVT with unfractionated heparin, the currently available low-molecular-weight heparins and the heparinoid danaparoid. Anticoagulation with an agent such as warfarin (Coumadin) should be used for at least three months, although the optimal duration of therapy after a pulmonary embolism is unknown.
| Drug | Dosage* | Comments | |
|---|---|---|---|
| Unfractionated heparin | 5,000-U bolus (or 80 U per kg) IV, 1,300 U (or 18 U per kg) per hour IV | Monitor activated partial thromboplastin time; in heparin resistance, target anti-Xa level of 0.4 to 0.7 U per mL | |
| Low-molecular-weight heparin | |||
| Enoxaparin (Lovenox) | 1 mg per kg SC every 12 hours | For all: when monitoring is required, target anti-Xa level of 0.4 to 0.7 U per mL | |
| Dalteparin (Fragmin)† | 100 anti-Xa U per kg SC every 12 hours | ||
| Ardeparin (Normiflo)† | ? | ||
| Heparinoid | |||
| Danaparoid (Orgaran)† | 2,500-U bolus IV; then 400 U per hour IV for 4 hours; then 300 U per hour IV for 4 hours; then 200 U per hour IV | Use for heparin-induced thrombocytopenia; target anti-Xa level of 0.5 to 0.8 U per mL | |
| When giving SC, 20 U per kg every 12 hours | |||
Special problems in oncology patients with thrombosis include a high risk of recurrence, failure to resolve thrombosis promptly and perceived increased risk of bleeding. In some cancer patients, thrombosis may appear refractory to treatment, recurring or not completely resolving during warfarin treatment despite a therapeutic International Normalized Ratio (INR). In these circumstances, heparin is more effective than warfarin, and patients can receive long-term low-molecular-weight heparin at treatment dosages.
To avoid a perceived high risk of bleeding while on anticoagulant therapy, some physicians believe cancer patients, particularly those with central nervous system cancer, should not receive anticoagulation and recommend inferior vena cava filter insertion instead. A retrospective study on the course of patients with thrombosis and primary or metastatic brain cancer showed a 57 percent incidence of vena cava or filter thrombosis, recurrent venous thrombosis or postphlebitic syndrome in patients who received filters rather than anticoagulation.27 In contrast, no patients who received anticoagulation as the primary therapy or who failed filter therapy developed such complications once anticoagulated.
Other work confirms no increased risk of central nervous system hemorrhage in patients with brain cancer.28 Studies of bleeding during anticoagulation identify age, hypertensive ischemic cerebrovascular disease, renal insufficiency, serious heart disease and a history of gastrointestinal bleeding as important risk factors, but not cancer.29
In the absence of prospective studies, the best therapy is probably either therapeutic anticoagulation for cancer patients with thrombosis rather than filter insertion or adding anticoagulation to filter therapy as soon as it is not contraindicated.
Prevention
The use of thromboprophylaxis in high-risk patients is not controversial. Nonetheless, it is not universally practiced, even in patients without contraindications. The optimal type and duration of prophylaxis is controversial, however.
Patients who have undergone a hip or knee replacement are at highest risk of all medical and surgical patients and have the fewest venographically demonstrable clots when given low-molecular-weight heparin. Predischarge ultrasound examinations of legs without thrombosis symptoms are of no demonstrable value.11,30
Opponents of prolonged or intensive postoperative prophylaxis in these patients point out that 10 days of warfarin prophylaxis leads to a three-month thromboembolism rate of 2 percent and a fatal pulmonary embolism rate of only 0.2 percent.30 They question the clinical significance of asymptomatic venographic thrombosis. Although postdischarge prolonged (one-month), once-daily low-molecular-weight heparin prophylaxis reduces the rate of venographic thrombosis in patients who have had hip replacement surgery,31,32 it does not appear to have this effect in patients who have undergone knee replacement.33
Proponents of intensive prophylaxis point to its safety, minimal inconvenience and relatively low cost (although at this time it is more expensive than unfractionated heparin or warfarin treatment). Unlike warfarin prophylaxis, intensive prophylaxis with low-molecular-weight heparin does not require monitoring. Both drugs require minimum patient cooperation beyond dosing, unlike pneumatic compression devices, which do not work when they are not worn and which interfere with ambulation when they are worn.
The use of compression devices as early prophylaxis is warranted in patients undergoing major pelvic surgery who, although at high risk for thrombosis, may34 or may not35 be prone to develop lymphoceles while receiving anticoagulants. Otherwise, compression devices alone represent inferior prophylaxis for high-risk patients.
Standard unfractionated heparin, usually given in a dosage of 5,000 units administered subcutaneously two or three times per day, is probably satisfactory in most general medical patients.
A recent study of 184 patients suggested that patients who were undergoing up to one hour or more of outpatient arthroscopic knee surgery with a tourniquet (lateral collateral ligament repairs and others) are at risk for venographic (18 percent overall, 5 percent proximal) and clinically symptomatic (11 percent) thrombosis.36 Fatal pulmonary emboli in such patients have also been reported. Thus, these patients should be cautioned about the risk of thrombosis, postoperative symptoms should be taken seriously and those with prior thromboembolic disease or diagnosed clotting disorder should receive special consideration for prophylaxis when or if the bleeding risk is acceptable.
Certain oncology patients are at particularly high risk of developing thrombosis. Studies of stage II breast cancer patients show that the period of chemotherapy and the addition of tamoxifen to chemotherapy both increase thrombosis risk.
In one study, nearly 10 percent of chemotherapy patients receiving tamoxifen developed thrombosis.37 Postmenopausal women were at highest risk. Patients with stage IV breast cancer, brain tumors and abdominal adenocarcinomas also appear to be at high risk. Administering 1 mg of warfarin daily for six weeks to patients with metastatic breast cancer who were receiving chemotherapy and then adjusting the dosage (average dose: 2.6 mg) to achieve an INR of 1.3 to 1.9 reduced the thrombosis rate by 85 percent with no excess bleeding.38
In another study, the 90-day upper extremity venographic thrombosis rate was reduced from 38 percent to 10 percent in patients with indwelling central-venous catheters who received 1 mg of warfarin per day.39
Based on this evidence, the use of very-low-dose warfarin in ambulatory high-risk medical cancer patients or 1 mg per day of warfarin in patients with indwelling central venous catheters is recommended. For hospitalized cancer patients, low-molecular-weight-heparin or standard heparin prophylaxis is recommended.