Am Fam Physician. 2002;66(5):780-783
Clinical Scenario
A 24-year-old woman, gravida 2, para 1, who has no history of preterm labor, presents at 13 weeks' gestation for prenatal care. She has no complaint of vaginal discharge.
Clinical Question
Should you perform screening for and, if the results are positive, treat asymptomatic bacterial vaginosis (BV) during this pregnancy?
Evidence-Based Answer
No. Screening for and treating BV in pregnant women who are at low risk for preterm delivery does not lower the risk of preterm delivery. If this patient were at high risk for preterm delivery (i.e., a history of preterm delivery or underweight before pregnancy), it would be reasonable to screen for BV and treat if the results are positive.
Cochrane Abstract
Background. BV has been associated with poor perinatal outcome. Because the infection is amenable to treatment, identification and treatment during pregnancy may reduce the risk of preterm birth and its consequences.
Objectives. The objective of this review was to assess the effects of antibiotic treatment of BV in pregnancy.
Search Strategy. The authors searched the Cochrane Pregnancy and Childbirth Group trials register and the Cochrane Controlled Trials Register.
Selection Criteria. Randomized trials comparing one antibiotic regimen with placebo or no treatment, or comparing two or more alternative antibiotic regimens in pregnant women with BV were included.
Data Collection and Analysis. Trial quality assessments and data extraction were done independently by three reviewers. Study authors were contacted for additional information.
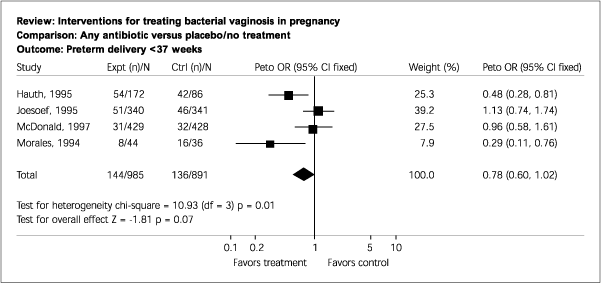
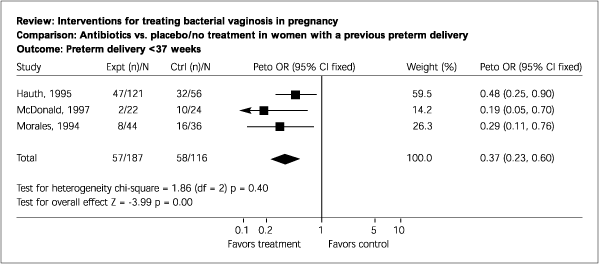
Primary Results. Five trials involving 1,504 women were included. These trials were of good quality. Antibiotic therapy was highly effective in eradicating infection during pregnancy, as judged by “test-of-cure” following therapy (odds ratio [OR], 0.22; 95 percent confidence interval [CI], 0.17 to 0.27). The effect of treating BV during pregnancy showed a trend toward fewer births before 37 weeks' gestation (OR, 0.78; 95 percent CI, 0.60 to 1.02). The prevention of preterm birth at less than 37 weeks' gestation was most marked in the subgroup of women with a previous preterm birth (OR, 0.37; 95 percent CI, 0.23 to 0.60).
Reviewer's Conclusions. The current evidence does not support screening and treating all pregnant women for BV to prevent preterm birth and its consequences. In women with a history of a previous preterm birth, there is some suggestion that detection and treatment of BV early in pregnancy may prevent a proportion of these women from having a further preterm birth. It is not known whether this outcome is associated with an improvement in neonatal well-being.1
These summaries have been derived from Cochrane reviews published in the Cochrane Database of Systematic Reviews in the Cochrane Library. Their content has, as far as possible, been checked with the authors of the original reviews, but the summaries should not be regarded as an official product of the Cochrane Collaboration; minor editing changes have been made to the text (http://www.cochrane.org)
Cochrane Critique
Did the authors address a focused clinical question ? Yes.
Were the criteria used to select articles for inclusion appropriate? Yes. They noted that one of the trials they included did not use an intention-to-treat analysis, which slightly weakened their conclusions.
Is it likely that important relevant articles were missed? No.
Was the validity of the individual articles appraised? Yes.
How precise are the results? Reasonably precise. The authors mentioned that only those data that were not heterogeneous were subjected to meta-analysis, but they did not explicitly describe the analyzed data as being non-heterogeneous.
Can the results be applied to patient care? Yes.
Do the conclusions make biologic and clinical sense? Yes.
Are the benefits worth the harms and costs? Although a formal cost-benefit analysis is not part of the Cochrane critique, it is reasonable to hope that, in patients with a history of preterm labor, screening for and treating BV may be cost-effective.2
Practice Pointers
In women who are not at high risk of preterm delivery, the evidence does not support screening for and treatment of asymptomatic BV. This statement is consistent with recent guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the U.S. Preventive Services Task Force (USPSTF).3,4
Since the publication of the Cochrane review,1 three clinical trials and one meta-analysis have been published that confirm this recommendation.3,5–7 In 2000, Kurkinen-Räty and colleagues6 reported finding no decrease in preterm delivery rates in 101 women who had been randomized to receive vaginal clindamycin or placebo.6 In a larger randomized controlled trial5 published in 2000,1,953 asymptomatic women from a general obstetric population were screened for BV and, if positive, treated with oral metronidazole or placebo. Carey and colleagues reported no reduction in rates of preterm labor or other outcomes. In 2001, Kekki and colleagues6 reported no effect of screening for and treating asymptomatic BV on preterm delivery in 375 healthy singleton women who were randomized to receive vaginal clindamycin or placebo.
In a quick calculation summing up the rates of preterm delivery from the three studies included in the Cochrane meta-analysis and the three new studies, the overall rate of preterm delivery is identical in antibiotic and control groups—12.7 percent. It will take another meta-analysis to properly compare these two, now enlarged, groups.
In high-risk women (i.e., those who have had a previous preterm delivery), some evidence supports screening for BV and treating only women with positive results. In this high-risk population, the number needed to treat can be calculated to be 5 using the data from the Cochrane review.1
Currently, ACOG recommends against screening and treating high-risk women.4 The USPSTF concludes that “a subgroup of high-risk women may benefit from screening and treatment; however, there may be a subgroup for whom BV treatment could increase the occurrence of preterm delivery.”3 Figures 1 and 2 summarize outcome with antibiotic therapy or placebo in all women and in those with a history of preterm labor.