
Am Fam Physician. 2003;68(5):889-897
Since the terrorist attacks of September 11, 2001, and the anthrax exposures in the following weeks, concern that smallpox could be used as a biologic weapon has increased. Public health departments and the U.S. military have begun the process of vaccinating soldiers and civilian first-responders. Smallpox vaccination carries some serious risks: approximately one in 1 million primary vaccinees and one in 4 million revaccinees will die from adverse vaccine reactions. The most serious side effects of smallpox vaccine include progressive vaccinia, postvaccinial central nervous system disease, and eczema vaccinatum. Some of these reactions can be treated with vaccinia immune globulin or cidofovir. Proper patient screening and site care are essential. Family physicians must learn to screen potential vaccinees for contraindications (e.g., immunodeficiency, immunosuppression, certain skin and eye diseases, pregnancy, lactation, allergy to the vaccine or its components, moderate or severe intercurrent illness) and to treat vaccine-associated adverse reactions.
Smallpox vaccine evolved from variolation, a technique that was developed in China and the Ottoman Empire. Variolation involved the deliberate exposure of nonimmune persons to material taken from known smallpox victims. Lady Montague, the wife of the British ambassador to the Ottoman Empire, observed variolation firsthand and advocated use of the technique in England. In 1798, the British physician Edward Jenner used a milkmaid's lymph containing cowpox virus to vaccinate a child.1
Because of the events of September 11, 2001, concern has risen that terrorist organizations or rogue states might use smallpox as a biologic weapon.4 In December 2002, the U.S. government began a smallpox vaccination program for civilian public health and hospital workers, including selected physicians, nurses, and ancillary personnel. The U.S. military also initiated a vaccination program for selected service members.
Smallpox Vaccine
Smallpox vaccine contains live vaccinia virus, a milder cousin of the variola (smallpox) virus.5 It does not contain smallpox virus and cannot cause smallpox. The vaccine contains lyophilized calf lymph and traces of polymyxin B, streptomycin, tetracycline, and neomycin.6 The vaccine diluent is 50 percent glycerin with a small amount of phenol.6 The vaccine does not contain egg byproduct or thimerosal.6
Once smallpox vaccine is reconstituted, it can be refrigerated and used for up to 60 days.7 After a single vaccination, 95 percent of patients are protected within 10 days,8,9 and immunity lasts at least five years (longer after revaccination).2,10 [Reference 8—Evidence level C, consensus/expert guidelines]
CONTRAINDICATIONS
| Eczema or atopic dermatitis (in the past, even if not currently active). Patients with these diseases or a history of these diseases should not be vaccinated. |
| Acute, chronic, or exfoliative skin conditions, including burns, impetigo, chickenpox, contact dermatitis, shingles, herpes, severe acne, Darier's disease (keratosis follicularis), and psoriasis. Until these conditions clear, patients should not be vaccinated. |
| Diseases or conditions that cause immunodeficiency or immunosuppression, including human immunodeficiency virus infection or acquired immunodeficiency syndrome, solid organ or stem-cell transplant, generalized malignancy, leukemia, lymphoma, agammaglobulinemia, and autoimmune diseases. Patients with these diseases or conditions should not be vaccinated. |
| Treatments that cause immunodeficiency or immunosuppression, including radiation therapy, antimetabolites, alkylating agents, corticosteroids, chemotherapy agents, and organ transplant medications. Patients should not be vaccinated until they, or their household contacts, have been off immunosuppressive treatment for three months. |
| Inflammatory eye diseases, including eye surgery and subsequent use of steroid eye drops. These patients should not be vaccinated until the ocular condition is well controlled and they have not been using steroid eye drops for at least two weeks. |
| Pregnancy, or plans to become pregnant in the next four weeks. Abstinence or highly effective contraceptive measures should be recommended to reduce the risk of pregnancy within four weeks of vaccination. |
| Previous allergic reaction to smallpox vaccine or any of the vaccine's components, including polymyxin B, streptomycin, tetracycline, neomycin, and phenol. Patients with such reactions should not be vaccinated. |
| Moderate or severe acute illness. Vaccination should be deferred until the illness has resolved. |
| Symptomatic or asymptomatic heart disease (previous myocardial infarction, angina, congestive heart failure, cardiomyopathy, stroke or transient ischemic attack, chest pain or shortness of breath on activity, or other heart conditions under the care of a physician) or cardiac risk factors (three or more cardiac risk factors: hypertension, diabetes, hypercholesterolemia, heart disease at 50 years of age in a first-degree relative, or current smoker). |
| Young or old age. Smallpox vaccination is contraindicated in children younger than 12 months. The ACIP advises against nonemergency use of smallpox vaccine in patients younger than 18 years or those older than 65 years. |
| Breastfeeding. Smallpox vaccine should not be given to women who are breastfeeding. |
| Latex allergy. Patients who are allergic to latex should not be vaccinated. Note that the vaccine vial stopper contains rubber. |
Steroid use is a contraindication to smallpox vaccination if the dosage of prednisone or its equivalent exceeds 2 mg per kg per day or 20 mg per day for two or more weeks.5 Patients must discontinue immunosuppressive treatment at least three months before they are vaccinated.6 Administration of smallpox vaccine is not contraindicated in patients who use nasal or oral steroid-containing inhalers or who receive soft tissue or joint injections containing steroids.
VACCINATION TECHNIQUE
Smallpox vaccine is given with multiple punctures of a bifurcated needle (Figure 1).13 The skin over the deltoid muscle or posterior arm is the most common vaccination site. If alcohol is used to cleanse the vaccination site, the site must be allowed to dry completely before the vaccine is administered.6
The bifurcated needle is held perpendicular to the skin and pressed rapidly into the skin three times for primary vaccination and 15 times for revaccination. Patients born before 1972 and patients who received smallpox vaccine more than 10 years ago should not be considered immune and should receive 15 punctures.2,6
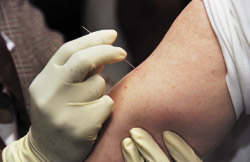
The needle should be pressed against the skin firmly enough to cause a trace of blood to appear at the site within 15 to 20 seconds.6 Excess vaccine is absorbed with sterile gauze, and the site is covered with gauze secured with tape.
CARE OF VACCINATION SITE
After vaccination, care must be taken to prevent autoinoculation or transmission to contacts. Vaccinees are infectious from about the third day after vaccine receipt until the scab falls off, which may take three to four weeks.14
Vaccinees should be educated not to touch the actual vaccine site or allow others to touch it. After intentional or accidental contact with the site, vaccinees should wash their hands with soap and water or an alcohol-based (more than 60 percent) hand rinse.11
Vaccinees' clothing, towels, and bed linen should be kept separate from those of unvaccinated contacts. While vaccinees have no restrictions on food preparation or travel, they should avoid public swimming or bathing.11
When health care workers are involved in patient care, they should cover their vaccination site with a semipermeable dressing and wear long-sleeved clothing to further reduce the risk of transmission. Vaccinated health care workers should assist each other with dressing changes. Medical leave is not required unless a health care worker develops debilitating symptoms or is unable to adhere to the precautions described above. Currently, there are no absolute restrictions on vaccinated health care providers interacting with infants, pregnant mothers, or immunosuppressed patients.11
Natural Course of Smallpox Vaccination
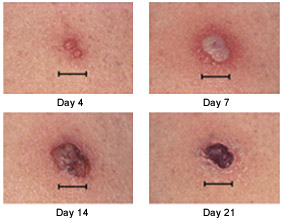
Responses to smallpox vaccine can be major or equivocal.15 The major response (Jennerian response or “take”) involves a two-to three-week progression from papule to vesicle to pustule to scab (Figure 2).16 All other responses are equivocal (“nontakes”). This vaccination process takes approximately 15 days in primary vaccinees and eight days in revaccinees.8,9,11
Normal reactions to smallpox vaccination include erythema, edema, regional lymphadenopathy, fever, malaise, and urticaria8 (Figure 3).17 These reactions require only observation and symptomatic treatment. In clinical trial settings, up to one third of vaccinees may be ill enough to miss work or have difficulty sleeping.8 Historically, approximately 21 percent of first-time vaccinees consulted a physician because of bothersome reactions.18 In contrast, recent data on military hospital workers demonstrated a 3 percent sick-leave rate.7,19
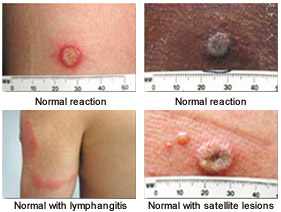
Approximately 10 percent of first-time vaccinees have “robust takes.”8 These reactions include large areas of erythema and extensive lymphadenopathy or lymphadenitis (Figure 4). It may be difficult to distinguish robust takes from bacterial infections. Robust takes occur eight to 10 days after vaccination and resolve spontaneously within 72 hours.8 No treatment is necessary.
If an equivocal response occurs, vaccination should be repeated with 15 punctures.6,11 A revaccinee should be considered immune if no take occurs after two attempts, while a primary vaccinee should be referred for immunologic evaluation if no take occurs after two attempts. Currently, there are no recommendations regarding the revaccination interval.
Smallpox vaccine may be administered simultaneously with any inactivated or live virus vaccine, except varicella vaccine.8 Smallpox and varicella vaccines should be administered at least 28 days apart to avoid confusing vaccine-related skin lesions.
Adverse Reactions to Smallpox Vaccine
Rates of adverse reactions to smallpox vaccine are lower in revaccinees.9,20 Data from the 1960s suggest that 1,000 of every 1 million vaccinees will have a serious reaction to the vaccine, and that approximately one in 1 million primary vaccinees and one in 4 million revaccinees will die.20,21 Rates of adverse reactions and death could vary considerably today. Although current populations may have a higher percentage of immunocompromised persons, such as those with HIV infection, significant advances have been made in the supportive care of critically ill patients.
Accidental implantation (autoinoculation) is the most common adverse reaction, occurring in approximately 600 of every 1 million vaccinees.18 The face, genitals, and rectum often are affected, and children are at highest risk.14 The best preventive measure is scrupulous hand-washing.14 Vaccinia immune globulin (VIG) is reserved for treatment in severe cases.
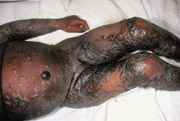
Patients with a history of eczema or atopic dermatitis, even if currently inactive, may develop eczema vaccinatum subsequent to smallpox vaccination (Figure 5).8 This condition occurs in 10 to 39 of every 1 million vaccinees and is sometimes fatal.18 Management includes intravenous hydration and meticulous skin care.8 High-dose VIG therapy has reduced the mortality rate for eczema vaccinatum from between 30 and 40 percent to 1 percent.20 [Evidence level C, consensus/expert guidelines]
Approximately three to 15 of every 1 million vaccinees develop postvaccinial central nervous system (CNS) disease.20 Postvaccinial encephalopathy usually affects vaccine recipients younger than two years and develops six to 10 days after vaccination, while postvaccinial encephalitis usually affects those older than two years and occurs 11 to 15 days after vaccination.8 Patients may present with headache, fever, vomiting, seizures, and coma. The mortality rate for this condition is 25 percent, and 25 percent of survivors have permanent neurologic deficits.20 Treatment is supportive, because VIG is not useful.8
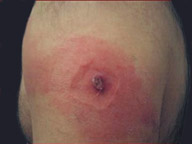
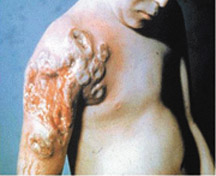
Progressive vaccinia only occurs in patients with a defective immune system. The condition involves unchecked viral replication, with progressive necrosis leading to severe viremia, shock, and death. Progressive vaccinia should be suspected in patients with vaccination sites that continue to progress beyond two weeks without apparent healing22 (Figure 6).8 High-dose VIG and cidofovir (Vistide) are the only known therapies.8,23
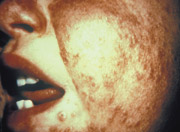
Occasionally, patients develop generalized vaccinia, in which viremia causes distant skin lesions (Figure 7).24 Generalized vaccinia usually occurs six to nine days after primary vaccination.22 Although visually distressing, this condition is typically benign and resolves spontaneously in one to two weeks. VIG may be used in patients with severe or recurrent vaccinia.8
Patients with inflammatory eye diseases are at risk for ocular vaccinial diseases, including blepharitis, iritis, and keratitis. Keratitis can lead to corneal clouding, ulceration and, possibly, blindness. Ocular vaccinial infections may respond to antiviral eye drops such as trifluridine (Viroptic) and to parenterally administered VIG.8 However, treatment with VIG is contraindicated in patients with keratitis, because this agent has been associated with the development of corneal opacities.9 Patients with ocular vaccinial disease should be treated in consultation with an ophthalmologist.
Fetal vaccinia is a rare complication of smallpox vaccination, with only 50 cases reported in the literature.8 The condition results from inadvertent vaccination during pregnancy or shortly before conception and leads to stillbirth or neonatal death. There is no reliable diagnostic test to confirm intrauterine infection. Pregnant women who have been inadvertently vaccinated should be referred to a perinatologist.
Although previously reported in vaccinees in Europe and Australia who received various smallpox vaccines, myopericarditis was reported rarely in vaccinees who received the New York Board of Health strain.19,25 Since January 2003, there have been more than 58 cases reported.19,25,26 It is unclear if this is truly an increase in incidence or simply better reporting.25 The exact cause is unknown. Patients typically present four to 30 days after vaccination with chest pain, electrocardiographic changes, elevated cardiac enzyme levels and, occasionally, abnormal echocardiograms. Cases have ranged from mild to severe.25 All patients have recovered fully with supportive care only.19,25,26
Rarely, the vaccine site may become secondarily infected, typically with staphylococci or streptococci. More commonly, a variety of nonspecific skin rashes, including erythema multiforme, may occur after smallpox vaccination. A recent trial27 describes vaccine-associated folliculitis as another possible cutaneous complication of smallpox vaccination.
Treatment of Adverse Reactions
VIG can be used in patients with extensive autoinoculation, eczema vaccinatum, severe or recurrent generalized vaccinia, or progressive vaccinia.8,11 VIG is not recommended for use in patients with mild autoinoculation, mild generalized vaccinia, erythema multiforme, or postvaccinial CNS disease. VIG therapy is contraindicated in patients with vaccinia keratitis.9
VIG without thimerosal is now available and meets the same standards as intravenous immune globulin. The usual dose is 0.6 mL per kg intramuscularly or 100 to 500 mg per kg intravenously.8 Adverse reactions to VIG include local site reactions, nausea, vomiting, fever, aseptic meningitis and, rarely, anaphylaxis.8 VIG is administered under an investigational drug protocol and can be obtained only through the CDC or the U.S. military.
Cidofovir has been approved for use under an investigational drug protocol. The drug is licensed for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. Cidofovir has in vivo and in vitro activity against vaccinia virus.8 Cidofovir never has been used to treat vaccinia virus infections in humans. This agent is reserved for the treatment of patients who do not respond to VIG or are near death.8 Cidofovir usually is given in a single intravenous dose of 5 mg per kg over 60 minutes. If clinically indicated, the dose may be repeated after one week. Patients treated with this agent should be monitored for renal toxicity.
Documentation and Reporting
Adverse reactions to smallpox vaccine should be reported to the Vaccine Adverse Event Reporting System (VAERS). Lost work time in excess of 24 hours and any need for hospitalization also should be reported. Reports can be submitted to VAERS via the Internet (vaers.hhs.gov) or by telephone (800-822-7967). Further information, including requests for VIG or cidofovir, can be obtained from the CDC Clinician Information Line (877-554-4625). Web sites for further information on smallpox vaccine are provided in Table 3.
Current Experience with Smallpox Vaccine
From January through July 2003, more than 38,000 U.S. civilians and more than 450,000 U.S. military personnel received the smallpox vaccine. As of July 25, 2003, the CDC reported three cases of generalized vaccinia, 18 cases of autoinoculation, 21 cases of myopericarditis, three cases of ocular vaccinia, and one case of postvaccinial encephalitis. Only one patient received VIG.26
The military experience through June 25, 2003 is notable for 36 cases of generalized vaccinia, one case of erythema multiforme, 48 cases of autoinoculation, 37 cases of myopericarditis, and one case of postvaccinial encephalitis. Two patients received VIG.19
Neither the CDC nor the U.S. military have reported any cases of eczema vaccinatum, fetal vaccinia, or progressive vaccinia.19,26 All cases of generalized vaccinia and myopericarditis have occurred in primary vaccinees.19,26 Overall, there have been fewer adverse reactions during this new vaccination period than what was predicted using historical data.19,25
| Portal to all CDC information, including vaccination tallies and adverse event reports:www.bt.cdc.gov/agent/smallpox/index.asp |
| Morbidity and Mortality Weekly Report Web site, which includes Smallpox Vaccination and Adverse Reactions: Guidance for Clinicians, as well as tally of reported adverse events:www.cdc.gov/mmwr |
| Official Department of Defense Web site, with in-depth smallpox information and vaccination resources:www.vaccines.army.mil/smallpox.asp |
| Smallpox overview, with Web links:www.mayoclinic.com/invoke.cfm?id=DS00424 |
| Walter Reed National Vaccine Healthcare Center Network (CDC and Department of Defense Web site for persons with health concerns about immunizations in the military health care system/TRICARE):www.VHCinfo.org |
| Vaccine Adverse Event Reporting System, to report problems associated with smallpox vaccination:vaers.hhs.gov |
Although there have been no deaths directly attributable to the smallpox vaccine, the CDC has reported five ischemic cardiac events following smallpox vaccination, with two deaths, and the U.S. military has reported one death from myocardial infarction five days after vaccination.19 These events, along with the number of myopericarditis cases, have led to revised screening recommendations for patients with known cardiac disease, vessel-related conditions, or other risk factors.