
Am Fam Physician. 2003;68(10):1992-1999
The 2001 Bethesda System for reporting cervical or vaginal cytologic diagnoses is an incremental change in the uniform terminology introduced in 1988 and revised in 1991. The 2001 Bethesda System includes specific statements about specimen adequacy, general categorization, and interpretation and results. In the adequacy category, “satisfactory” and “unsatisfactory” are retained, but “satisfactory but limited by” is eliminated. The new category of “atypical squamous cells” (ASC) replaces the category of “atypical squamous cells of undetermined significance” (ASCUS) and is divided into qualifiers of (1) ASC of “undetermined significance” (ASC-US) and (2) “cannot exclude high-grade squamous intraepithelial lesion (HSIL),” or (ASC-H). The categories of ASCUS, “favor reactive” and “favor neoplasia” are eliminated. The terminology for low-grade squamous intraepithelial lesions (LSILs) and HSILs remains unchanged. The category of “atypical glandular cells of undetermined significance” (AGUS) is eliminated to avoid confusion with ASCUS and is replaced by the term “atypical glandular cells” (AGC), with attempts to identify whether the origin of the cells is endometrial, endocervical, or unqualified. “Endocervical adenocarcinoma in situ” and “AGC, favor neoplastic” are included as separate AGC categories. The presence of normal or abnormal endometrial cells is to be reported in women who are at least 40 years of age. Educational notes and comments on ancillary testing may be added as appropriate.
The Bethesda System (TBS) for reporting cervical or vaginal cytologic diagnoses was introduced in 19881 and revised in 19912 to establish uniform terminology and standardize diagnostic reports. In addition, it introduced a standardized approach for reporting if an individual specimen is adequate for evaluation. TBS 2001 was developed through a process that involved committee review of the literature, solicitation of expert opinions, and discussion of the proposed changes on an interactive Web site.3 [Evidence level C, consensus/expert guidelines] The terminology of TBS 2001,3 which was adopted in May 2001, includes revisions in statements of adequacy, general categorization, and interpretation and results of epithelial cell abnormalities (Table 1).3 The American Society for Colposcopy and Cervical Pathology Consensus Conference subsequently developed guidelines for the management of cervical cytologic abnormalities.4 [Evidence level C, consensus/expert guidelines]
Specimen Adequacy
TBS 19912 reported the adequacy of cervical cytology preparations in three categories: “satisfactory,” “unsatisfactory,” and “satisfactory but limited by,” or SBLB. SBLB included factors such as the lack of transformation zone components and the presence of partially obscuring factors (i.e., blood or inflammation). This category was confusing to some clinicians and prompted unnecessary repeat testing.
It has been shown that the presence of endocervical cells as a quality indicator of adequate sampling increases the detection of cervical abnormalities5; however, other studies6,7 have not demonstrated that a lack of transformation zone components in otherwise negative specimens indicates a higher risk of subsequent detection of histologic high-grade squamous intraepithelial lesions (HSIL). Lack of endocervical cells has not been shown to be associated with an excess of disease in longitudinal studies in which histologic disease, rather than cytologic prediction of disease, is the end point.8
Partially obscuring factors also have not been shown to increase the risk for a false-negative report.9 In TBS 2001,3 the SBLB category is eliminated, and comments about transformation zone components or partially obscuring factors are placed in the satisfactory or unsatisfactory categories as a means of providing feedback to improve specimen adequacy10 (Table 2).1,3 Because specimens lacking a transformation zone component now will be reported as “satisfactory for evaluation,” clinicians should read the narrative report carefully to learn that the transformation zone was not sampled.
| Eliminated |
| Satisfactory but limited by |
| Benign cellular changes (as a separate category) |
| Atypical squamous cells of undetermined significance (ASCUS), favor reactive |
| ASCUS, favor neoplastic |
| Atypical glandular cells of undetermined significance (AGUS), favor reactive |
| AGUS, favor dysplasia |
| Hormonal evaluation |
| Added |
| “Other” category to include endometrial cells in women at least 40 years of age |
| Atypical glandular cells (AGC) |
| AGC, favor neoplastic |
| Endocervical adenocarcinoma in situ |
| Atypical squamous cells of undetermined significance (ASC-US) |
| Atypical squamous cells, cannot exclude highgrade squamous intraepithelial lesion (ASC-H) |
| Automated review |
| Ancillary testing |
| Other modifications |
| Renaming “within normal limits” to “negative for intraepithelial lesion or malignancy” |
| Organisms and other non-neoplastic findings optional under “negative for intraepithelial lesion or malignancy” |
Merely eliminating SBLB will not change the cytologic appearance of the specimens. This, in conjunction with the introduction of specific numeric criteria for the number of cells that must be present on a slide for it to be classified as satisfactory for evaluation, means that the rate of unsatisfactory specimens likely will increase significantly.11
The unsatisfactory category includes specimens that do not contain sufficient cells for reliable interpretation. However, any specimen with abnormal cells will be described as satisfactory for evaluation regardless of the number of cells present.3 This does not mean that an unsatisfactory specimen reflects the absence of a neoplastic process. Although an unsatisfactory specimen can represent a benign condition, a considerable number of women with unsatisfactory specimens have a subsequent histologic diagnosis of squamous intraepithelial lesion (SIL) or cancer.12
General Categorization
In TBS 2001, cervical cytologic specimens that contain no epithelial abnormalities are listed under the category “negative for intraepithelial lesion or malignancy.”3 This category now encompasses the previous categories of “within normal limits” and “benign cellular changes”13(Table 2).1,3 The presence of organisms (listed as “infections” in TBS 19912) such as Trichomonas vaginalis or fungal organisms consistent with Candida species will be included as a comment in this “negative” category. Components that are optionally listed in the negative category include atrophy, radiation, and inflammation.
Interpretation/Result
EPITHELIAL CELL ABNORMALITY, SQUAMOUS
Atypical Squamous Cells
Since TBS 1988,1 the category of “atypical squamous cells of undetermined significance” (ASCUS) has included cells for which a reliable interpretation of SIL cannot be made although the cells contain features that are more marked than merely reactive changes.14 Subcategories of ASCUS were not used in TBS 1991,2 but in an attempt to define risk, it was suggested that qualifiers such as “ASCUS, favor reactive,” or “ASCUS, favor neoplastic” could be used. However, no consensus was reached on how to define each subcategory, and numerous studies showed that the use of these qualifiers was nonreproducible.15
In addition, several studies demonstrated that the diagnosis of ASCUS cannot be ignored. A study16 of 4,143 diagnoses of ASCUS with subsequent histology reported that in 63 percent of the women, SIL or malignancy was detected on further follow-up. In a study17 correlating cervical cytology and subsequent histology in 560 women with ASCUS, 17 percent of the follow-up biopsies demonstrated HSIL, and 19 percent showed a low-grade squamous intraepithelial lesion (LSIL).
Another study of more than 46,000 women receiving routine cervical cytologic screening demonstrated that the most common cytologic result immediately preceding the diagnosis of histologic HSIL or greater was ASCUS (39 percent of all cases of HSIL or cancer).18
Although ASCUS was a problematic category because of poor interobserver reproducibility,19,20 there was strong support to maintain an equivocal category in TBS 2001,3 based on studies indicating that ASCUS is an important contributor to high-grade cervical disease. Complete elimination of the ASCUS category would result in some histologic high-grade lesions being classified as normal cervical cytology.
In TBS 2001,3 the new category of “atypical squamous cells”(ASC) emphasizes the importance of determining risk status by dividing smears into likelihood of being “negative” or harboring an SIL.14 ASC was divided into two subcategories: atypical squamous cells “of undetermined significance” (ASC-US) and atypical squamous cells, “cannot exclude HSIL,” or ASC-H. TBS 20013 eliminates ASCUS, “favor reactive” with the intent that most of the specimens in this category will be downgraded to “negative.”
In the TBS 20013 classification system, ASC-US is the more numerically prominent qualifier and should account for 90 to 95 percent of all ASC results (Figure 1). The use of the qualifier “undetermined significance” emphasizes that a specific diagnosis cannot be made and that further triage may be appropriate. ASC-US will include most cytology results previously categorized as “ASCUS, not otherwise specified” (ASCUS-NOS) or “ASCUS, favor SIL.” ASC-US excludes cytology suggestive of HSIL. ASC-H is interpreted as cytologic changes that are suggestive of HSIL but lack criteria for definitive interpretation (Figure 2).3 ASC-H is the less common qualifier, accounting for 5 to 10 percent of all ASC cases, but the risk of an underlying high-grade lesion is higher in this category than in ASC-US.21 The positive predictive value for HSIL (cervical intraepithelial neoplasia [CIN] 2, 3) in ASC-H is higher than in the category ASC-US but not as high as in the category HSIL.22
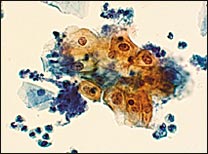
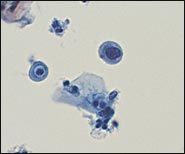
Low-Grade Squamous Intraepithelial Lesion
The category of LSIL is unchanged in TBS 20013 and continues to include the following categories: human papillomavirus (HPV) (Figure 3), mild dysplasia, and CIN 1. The debate at TBS 20013 concerning CIN 2 being included either in LSIL or HSIL resolved with the decision that the dividing line between LSIL and HSIL would remain between CIN 1 and CIN 2. This decision reaffirmed the two-tiered approach of LSIL and HSIL in previous TBS terminology.3 It has been shown that there is less reproducibility for LSIL than for HSIL,23 and that the rate of LSIL is more variable than the rate of HSIL.24 The accuracy rate of interpretation of LSIL is approximately 80 percent.25 Of the 20 percent that are misclassified, almost one third are actually a higher grade lesion.
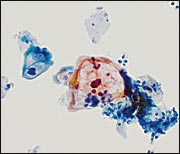
High-Grade Squamous Intraepithelial Lesion
EPITHELIAL CELL ABNORMALITY, GLANDULAR
TBS 198814 used the term “atypical glandular cells of undetermined significance” (AGUS) to describe “cells showing either endometrial or endocervical differentiation displaying nuclear atypia that exceeds obvious reactive or reparative changes but lack unequivocal features of invasive adenocarcinoma.”14 It was suggested that qualifiers such as “favor reactive” and “favor neoplastic” could be used. The spectrum of AGUS ranged from benign-appearing reparative findings to adenocarcinoma in situ.
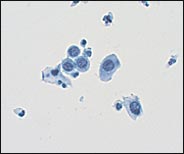
Several problems have arisen from the use of the term AGUS. Clinicians have been confused about the differentiation of AGUS and ASCUS, creating the risk that the benign-sounding, but more problematic, AGUS may be undermanaged. A study26 indicates that the term AGUS is a misnomer—rather than being a vague cytologic category, it actually represents a marker for significant glandular or squamous pathology. As many as 44 percent of women diagnosed with AGUS actually may be found on follow-up to have a squamous lesion.27 In the previous study,26 women in the category AGUS with a coexisting squamous abnormality were 9.7 times more likely to have histologic CIN 3 than women in the category AGUS, favor reactive.
In another study,28 the overall positive predictive value for cervical cytology specimens with a glandular abnormality was approximately 73 percent for either significant glandular or squamous pathology and 56 percent for significant glandular pathology alone. The sensitivity of the cervical cytology for the diagnosis of adenocarcinoma and preinvasive glandular lesions was 45 to 76 percent.29 One study30 found that the false-negative rate in women with known cervical adenocarcinoma was 42 percent.
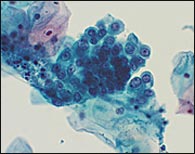
TBS 20013 recommends that the term AGUS be replaced with the term “atypical glandular cell” (AGC) and that the “favor reactive” qualifier be eliminated.3 The laboratory will attempt to indicate whether the origin of the AGC is endocervical, endometrial, or unqualified (Figure 5). In addition, adenocarcinoma in situ (a new term in TBS 20013) and “AGC, favor neoplastic,” are listed as separate categories.
OTHER
TBS 20013 designates an “other” category for reporting normal or abnormal endometrial cells in women who are 40 years or older.3 The presence of even benign-appearing endometrial cells on cervical cytology in women who are at least 45 years of age is more often associated with endometrial adenocarcinoma and endometrial hyperplasia than with benign endometrium.31 However, according to TBS 2001,3 “cervical cytology is primarily a screening test for squamous epithelial lesions and squamous cancer. It is unreliable for the detection of endometrial lesions and should not be used to evaluate suspected endometrial abnormalities.”3
EDUCATIONAL NOTES AND ANCILLARY TESTING
In TBS 1988,1 the Papanicolaou test was viewed as a “consultation” between the cytopathologist and the clinician, thus encouraging the inclusion of comments on management. However, these educational comments were often misleading, because evidence-based management guidelines were unavailable and the cytopathologist usually did not know the patient's clinical history. Therefore, clinicians were unclear if they were obliged to follow the recommendations in the notes. TBS 20013 recommends that the use of optional written comments regarding the interpretation of a cytologic specimen be conveyed to the clinician as a means of clarification and information.3 However, when discussing issues, the 2001 Consensus Guidelines for the Management of Women with Cervical Cytological Abnormalities4 should be used when possible.