
Am Fam Physician. 2004;70(6):1071-1078
Gait instability, urinary incontinence, and dementia are the signs and symptoms typically found in patients who have normal pressure hydrocephalus. Estimated to cause no more than 5 percent of cases of dementia, normal pressure hydrocephalus often is treatable, and accurate recognition of the clinical triad coupled with radiographic evidence most commonly identifies likely responders. Magnetic resonance imaging or computed tomography typically demonstrates ventricular dilation with preservation of the surrounding brain tissue. The abnormality in normal pressure hydrocephalus occurs secondary to an abnormality in fluid removal, leading to an increase in ventricular size and encroachment of enlarged ventricles on adjacent brain tissue. The pressure exerted on the cerebral parenchyma by immense fluid-filled cavities deforms white matter tracts, instigating gait abnormalities and incomplete control of the bladder, as well as difficulties in processing incoming stimulation and in producing expeditious responses. Signs and symptoms often occur as sequelae to an imbalance between the expected ongoing production of cerebrospinal fluid and continuous efflux. Ventriculoperitoneal shunting is used to relieve excess ventricular fluid not absorbed by normal physiologic channels. Multiple studies have explored various techniques to identify patients with normal pressure hydrocephalus in an effort to predict likely benefit from shunting. However, the effectiveness of cerebrospinal fluid diversion has never been proven in a randomized controlled trial comparing use of a shunt versus no shunt.
The diagnosis of normal pressure hydrocephalus (NPH) depends on symptom profile, presence of radiographic features, and the outcome of diagnostic tests. Although numerous techniques are used to identify patients who are likely to have NPH and various means are used to identify those patients most likely to respond to treatment, no definitive method exists to prove diagnosis. Cerebrospinal fluid (CSF) diversion accomplished via placement of a ventriculoperitoneal shunt is the most common treatment.
The precise incidence of NPH is hard to determine, because the condition lacks a formal, consensus-based definition. Some physicians base the diagnosis strongly on radiographic evidence; another group of health care professionals relies more on clinical indications, and still others use a combination of signs and symptoms that they have found to be reliable. A rare cause of dementia, NPH primarily affects persons older than 60 years and is estimated to be the source of dementia in 5 percent or less of affected persons.1–3
Background
Formed by specialized tufts of capillaries called choroid plexus at a rate of approximately 20 mL per hour, CSF circulates from dual paired lateral ventricles through paired foramina of Monro into a single midline third ventricle (Figure 1). From this midline cavity, CSF continues through the aqueduct of Sylvius into the fourth ventricle, situated within the posterior fossa. CSF exits the ventricular system via three small apertures to pass into the subarachnoid space. CSF contained within the subarachnoid space envelops and cushions the brain and spine. At any one time, 140 mL of CSF is contained within the neuroaxis; approximately 25 mL is contained within the ventricles while the majority is transiently sequestered within cisternae situated at the base of the brain or within spaces surrounding the cerebral convexities and the spinal cord. Within the subarachnoid space, CSF is absorbed by arachnoid granulations positioned near the top of the brain. These conduits of CSF outflow drain into the venous system via the superior sagittal sinus.2,4
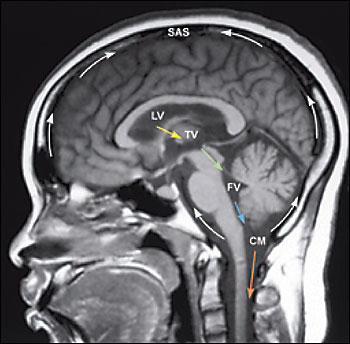
NPH is a disorder of decreased CSF absorption, not excess formation.4 Whether from an established or unknown cause, the arachnoid granulations fail to maintain their baseline removal of CSF, often secondary to fibrosis and scarring that obscure absorptive interfaces. A pressure gradient develops between the fluid in the subarachnoid space surrounding the brain and the ventricular system. The differential pressures eventually lead to decreased CSF production and the setting of a higher, yet still normal, baseline pressure.1 This new pressure distends the ventricles, stretching surrounding nerve fibers and compressing the periventricular parenchyma. This encroachment on brain tissue by enlarged ventricles impinges on the caliber of arterioles and capillaries, often resulting in ischemia.2
The theory that implicates fibrosis as the pathophysiologic basis of NPH is not uncontested. Some sources believe that idiopathic NPH is a persistence of poor drainage of CSF originating in childhood as benign external hydrocephalus secondary to insufficient removal of CSF by immature arachnoid granulations.5,6 Additionally, agreement about whether NPH is an entity consisting of normal, or near normal, pressure remains far from unanimous because some physicians feel that symptoms may be the result of intermittent spikes of high pressure known as B waves.1,7–10
Regardless of the originating cause, a communicating hydrocephalus is produced. This type of hydrocephalus, in contrast to non-communicating hydrocephalus, indicates the absence of an obstructive mass, such as a tumor, abscess, or blood clot, infringing on the central pathway of CSF flow.
The inciting etiology of NPH may be recognized or unknown. Subarachnoid hemorrhage, head injury, and meningitis are frequently implicated preceding events.3,4 Patients with established etiology of NPH tend to respond more favorably to shunting than patients with chronic idiopathic hydrocephalus.3,5,11–13
Signs and Symptoms
The triad of gait instability, urinary incontinence, and dementia distinguishes NPH4,14–16 (Table 1). Elucidation of the presence of these three symptoms, as well as their predominance and pattern of presentation, is essential. One study2 reported a 65 percent positive predictive value of this classic constellation in selecting patients for ventriculoperitoneal shunting.
| Ambulation | |
| Difficulty or arrest in initiation of ambulation | |
| Feet appear “glued to the floor” | |
| Gait instability, multiple falls Wide-based stance, shuffling steps | |
| General | |
| Generalized slow movement | |
| Incontinence | |
| Mentation | |
| Lack of spontaneity in movement, verbal response, and emotion | |
| Latency in response to questions or reaction to situations | |
| Slowness in processing information | |
Although NPH commonly is referred to as a treatable form of dementia, cognitive deficits and memory loss are the symptoms least specifically indicative of this syndrome and the last to respond to shunting.15,17,18 Gait instability is most often the first presenting and most significant problem.7,12,16,19,20 In a review20 of 35 studies evaluating diagnostic studies and outcomes of patients with NPH, investigators arrived at a similar conclusion. The aberrant pattern of ambulation often is composed of a slow gait; short, shuffling steps; and a wide-based stance.4,15,21
The term “gait apraxia” is sometimes used to describe the problem with locomotion experienced in patients with NPH. This phrase entails difficulty in sequencing the individual components that constitute ambulation (i.e., strike, stance, and swing), as well as a struggle to coordinate alterations in course or fluid continuity of movement. Indeed, trouble with initiation of ambulation, imbalance during action and standing, as well as frequent falls, are observed.7 In one study,16 10 patients withNPH were compared with 12 healthy, age-matched control patients; the patients with NPH were found to have a slower gait, shorter stride, and imbalance offset by a wide stance.
Urinary incontinence usually follows gait abnormalities and almost always includes urinary urgency.1,2,22 Deformation of periventricular corticospinal tract sacral nerve fibers seems to be the likely reason for incontinence.4 Ineffective contraction of the detrusor muscle is identified on urodynamic studies.4
Dementia usually is not the presenting or most overt symptom of NPH.18 It is often, although not invariably, evident.1,4 If present, cognitive impairment should be of the subcortical type. Inattention, latency in recall, and lack of spontaneity frequently are encountered indicators of subcortical dementia, the intellectual impairment observed in NPH.4 An answer provided by a patient suffering from NPH is frequently correct; a fact recalled is most often accurate.1 These features distinguish the dementia of NPH from the cognitive decline noted in patients with Alzheimer’s disease.12 NPH dementia should not include difficulty with word formation, trouble interpreting stimuli within appropriate context, or inability to sequentially carry out simple tasks, which characterize aphasia, agnosia, or apraxia and are more indicative of cortical dementias. The physiologic cause of the dementia intermittently associated with NPH remains to be determined positively, although deformation of limbic structures surrounding the enlarged ventricles has been suggested.4
Ultimately, identifying patients with NPH involves the careful weighing of likelihoods. The time to refer patients to a subspecialist (i.e., neurologist or neurosurgeon) for a more involved and specific work-up and confirmation or contradiction of suspicion is when even a slight chance of the diagnosis arises or when just a loose grouping of the triad is encountered. Early referral, when NPH is suspected, is optimal to identify the greatest number of patients actually experiencing this disorder and to offer timely treatment to those who are most likely to respond to intervention. Expedient differentiation of NPH from other diagnoses, some of which are life-threatening, as well as directly and conclusively treated, also is imperative (Table 2).
| Alzheimer’s disease |
| Carcinomatous meningitis |
| Chronic alcoholism |
| Combinations of conditions affecting gait (e.g., rheumatoid arthritis, cervical stenosis) coupled with conditions that impinge on mentation (e.g., Alzheimer’s disease, multi-infarct dementia) and those that have an effect on urination (e.g., prostate disease) |
| Intracranial infection (e.g., abscess, subdural empyema, meningitis) |
| Multi-infarct dementia |
| Parkinson’s disease |
| Subcortical arteriosclerotic disease |
| Subdural hematoma |
| Systemic diseases (e.g., hypothyroidism, Addison’s disease) or malignancy |
| Tumor (benign or malignant) |
Radiographic Features
Imaging is essential in patients presenting with signs and symptoms of NPH to confirm the absence of subdural hematoma, infection, neoplasia, or other structural abnormality. Compared with studies of normal patients (Figure 2a), magnetic resonance imaging (MRI) of patients who have NPH demonstrates ventriculomegaly with maintained cerebral parenchyma (Figure 2b). This finding is in contrast to the ventricular dilation associated with significant loss of brain tissue evident in images of patients who have Alzheimer’s disease (Figure 2c). Additionally, MRIs of patients who have Alzheimer’s disease frequently reveal focal loss of tissue in the hippocampus.1 In patients with NPH, the volume of medial temporal lobe tissue should be maintained.1
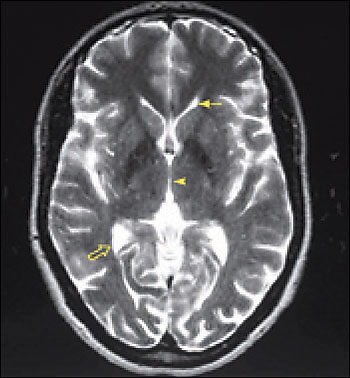
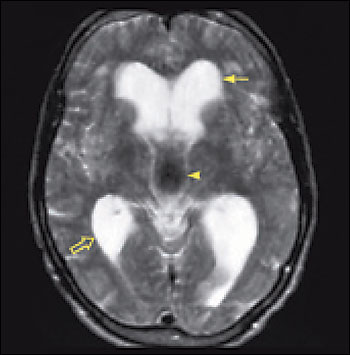
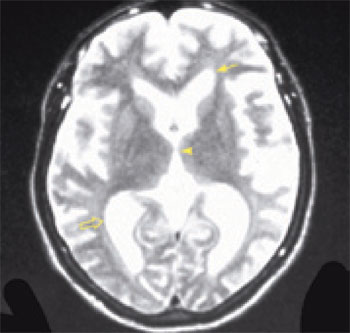
Evidence of turbulent flow in the posterior third ventricle and within the aqueduct of Sylvius may increase the likelihood of shunt-responsive NPH.1,4,23–25 Magnetic resonance flow imaging—called cine MRI—helps to define and characterize this flow void. CSF flow studies may help differentiate NPH from atrophy.25
Radionuclide cisternography may suggest the presence of communicating hydrocephalus via stasis of isotope within the ventricles. Demonstration of a sluggish rate of isotope travel to the convexities in 48 hours may be used to confirm communicating hydrocephalus, but should not be used to exclude otherwise favorable patients from the shunting procedure.4
White matter disease is observed frequently in brain parenchyma adjacent to enlarged ventricles, coupled with diminished perfusion evident on flow studies. Perfusion to the periventricular region actually may increase following shunt surgery.5
Diagnostic Studies
Five diagnostic studies can be used to assess patients who may have NPH. These include routine spinal tap, high volume spinal tap, prolonged lumbar drainage, continuous intracranial monitoring, and CSF outflow evaluation.
ROUTINE SPINAL TAP
Routine spinal tap should be performed to rule out other conditions. A normal level of CSF protein and glucose, and a white blood cell count of five or fewer cells per mcL [or mm3] plus an opening pressure of less than200 mm H2O suggests that NPH may be thecause of the neurologic symptoms.
INTERMITTENT HIGH VOLUME TAP
In an intermittent high volume tap, 30 to 60 mL of CSF are removed with comparison of symptoms before and after the test. Improvement of symptoms with careful removal of 30 to 60 mL of CSF may indicate an eventual positive response to ventriculoperitoneal shunting.4,19,26 High volume tap coupled with symptom assessment can be repeated to confirm the reproducibility of positive or negative results. In one study26 of patients who underwent this test, 80 percent of patients who showed symptom improvement following a high volume tap had a positive response to shunting at three months, with 73 percent reporting benefit at three years.
PROLONGED LUMBAR DRAINAGE
Some authors find three to five days of lumbar drainage via interval or constant pump-controlled removal of CSF combined with ongoing assessment of the patient’s condition to be more indicative of eventual response to CSF diversion.27,28 Five to six days of CSF diversion via lumbar drain proved 100 percent effective in predicting positive outcome to shunting in two series of seven and 17 patients.27,28
INTRACRANIAL PRESSURE
Although “normal pressure” is inherent in the name of this syndrome, some evidence obtained from monitoring intracranial pressure over an extended period of time may reveal intermittent spikes of elevated pressure. Placement of an intracranial pressure monitor allows for a period of continuous recording of intracranial pressure, permitting identification of episodic waves of high pressure that are missed with isolated lumbar measurement yet are often characteristic of NPH.1,7–10
CSF OUTFLOW STUDIES
CSF outflow studies examine the pressure response to outflow following infusion of a sterile solution at rates of 0.5 mL per minute to 5 mL per minute.7 This test requires access to the lumbar cistern via lumbar puncture for infusion of fluid and placement of a ventriculostomy for measurement of intracranial pressure monitoring during the test as well as the release of CSF once the intracranial pressure has reached a certain level.7 In some studies,29,30 elevated outflow resistance predicted which patients would be most likely to respond favorably to shunting. However, in reviewing the diagnostic methods and outcomes from a review20 of 35 studies of NPH cohorts, investigators report inconsistent results from measurements of CSF outflow pressure.
Ultimately, there is a lack of consensus about which diagnostic test most reliably predicts which patients will respond favorably to shunting. No gold standard test is available to identify patients who will benefit from CSF diversion. In the absence of agreement among experts, the outcomes of various or multiple tests coupled with the sequence and significance of signs and symptoms together with results of radiographic studies identify patients who likely have NPH.
Treatment
Ventriculoperitoneal shunting remains the most common therapy for NPH. This procedure involves the use of general anesthesia. A catheter is placed into one lateral ventricle and attached to a cap and valve positioned below the scalp. Tubing is tunneled subcutaneously from the valve to the abdomen, where it is deposited into the sterile peritoneal cavity for continuous drainage.
Examination of the results of multiple studies yields a wide variation in patient response to CSF diversion. According to one review,20 59 percent of patients with NPH reported improvement after shunting with a persistent improvement in only 29 percent. In another study,7 at one year following ventriculoperitoneal shunting in their cohort of 25 patients, 72 percent of patients had improvement in activities of daily living, a 58 percent improvement in urinary incontinence, and a 57 percent improvement in ambulation.
Final Comment
Patients with a known cause of NPH are more likely to have improvement from ventriculoperitoneal shunting than those who lack a defined etiology. Signs or symptoms of short duration are more likely to be ameliorated with CSF diversion than longstanding impairment. Patients whose symptoms improve following a large volume lumbar puncture have a greater chance of responding to shunting than patients whose deficits remain unchanged following removal of CSF.
A patient presenting primarily with gait instability, followed by incontinence and mild dementia of short duration, with imaging showing ventricular dilation but a preserved cortical mantle, has a reasonable likelihood of benefiting from ventriculoperitoneal shunting. A floridly demented patient whose ineffective ambulation and incontinence likely are secondary to cognitive decline accompanied by radiography demonstrating significant, generalized cortical atrophy will not likely respond to CSF diversion.17,18
The decision to shunt should not be taken lightly. Accurate patient selection is key to a successful outcome. If symptoms involving gait, incontinence, and/or dementia are present for a short period of time, patients have a higher chance of amelioration of these symptoms with shunting than patients who have had symptoms for a long time.1 However, improvement has been recognized in patients even after years of cognitive decline. Therefore, treatment should not be withheld, if otherwise indicated, solely on the basis of symptom duration. Yet, with NPH, no strict algorithm or definitive test provides an ironclad answer. In the absence of absolute proof remain practical indications and clinical likeliness to formulate decisions.