
Am Fam Physician. 2006;74(5):783-790
Author disclosure: Nothing to disclose.
Avian influenza A (H5N1) first emerged as a global public health threat in 1997 when it caused a major human outbreak in Hong Kong. Endemic in waterfowl and highly virulent in poultry, H5N1 is capable of incidentally infecting humans and other mammals. Although H5N1 is not yet capable of efficient human-to-human transmission, the protean nature of its genome could transform it into the source of the next human influenza pandemic. In the spring of 2006, migrating birds spread the virus from Asia to Europe and Africa. Preparing for a new influenza pandemic involves increasing global influenza surveillance and developing practical strategies for containing outbreaks at the source. Prompt case recognition, isolation, and treatment will be crucial for disease control. Pharmacologic interventions will focus on streamlining the production of vaccine, extending vaccine supplies, stockpiling antiviral drugs such as oseltamivir, and distributing these agents in a timely manner to persons who have the most need. Nonpharmacologic measures will include the use of masks, social distancing, quarantine, travel restrictions, and increasing the emergency capacity of health care systems.
Avian influenza has emerged as the primary public health concern of the 21st century. Although various strains of avian influenza have been recognized for decades, the scope, lethality, and mutability of the Asian H5N1 subtype make it a likely source of the next human influenza pandemic—an event that could kill millions. H5N1 no longer is confined to waterfowl and poultry in southeast Asia and China and appears to be expanding its host and geographic ranges.1 As of late June 2006, 228 persons had been infected with H5N1, most of whom had close contact with infected birds; 130 of these persons died.2 Other mammals such as cats and pigs also have been infected. Waterfowl migration helped the virus spread into Europe and Africa, and it may not be long before it appears in North America. Preparing for this new threat has replaced eradication as a global public health priority.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Pharyngeal swabs are more effective than nasal swabs in diagnosing avian influenza A (H5N1) infection. | C | 14 |
| Oseltamivir (Tamiflu) can reduce the severity and duration of symptoms if treatment is initiated in the first 48 hours after symptom onset. | C | 23 |
| N95 particulate masks should help prevent person-to-person transmission of H5N1. Surgical masks prevent only large-droplet transmission. | C | 14,27 |
History of Avian Influenza
Avian influenza A virus is shed in the feces of healthy-appearing waterfowl (primarily ducks), which in turn infect chickens and other poultry with which they come in contact. Mortality rates in chickens and other birds can be devastating with highly pathogenic strains. Conditions for transmission and jumping species barriers often are ideal in Asia, where poultry, ducks, pigs, and humans live in crowded conditions. Although pigs were thought to be a necessary “bridge” species for avian viruses to infect humans, it now seems likely that the avian influenza virus is mutable enough to make the interspecies leap on its own.3
H5N1 first emerged as a human threat in Hong Kong in 1997. An outbreak in poultry infected 18 people, six of whom died; however, the disease seemed to vanish after the prompt culling of all poultry in Hong Kong.4 In 2003, additional cases were reported in Hong Kong, followed by serial outbreaks of the even more pathogenic “Z strain” of H5N1 in Thailand, Vietnam, Indonesia, Cambodia, and China in 2004 (Figures 15 and 26). As the virus became entrenched in wild bird populations throughout Asia, its 50 percent mortality rate in incidentally infected humans ensured “bird flu” a regular spot in news headlines. In 2006, H5N1 spread along migratory pathways to Turkey, where it caused a human outbreak, and to Russia; the virus then spread to Europe and several African nations. Human outbreaks of H5N1 infection in Asia have declined in 2006, but this trend could change anytime.
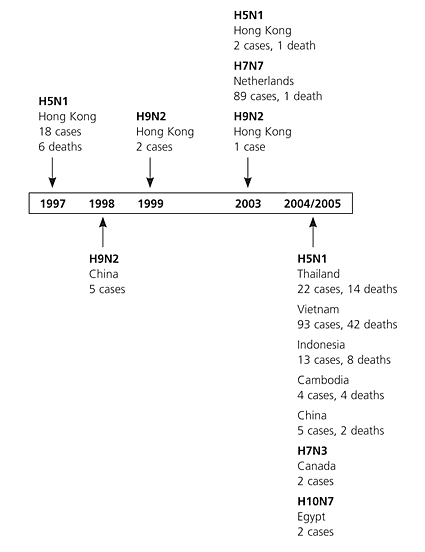
H5N1 is the most virulent subtype in a long list of highly pathogenic avian viruses that have emerged in recent years. H9N2 circulated in Asian poultry in 1998 and 1999, and a 2003 outbreak of H7N7 in the Netherlands caused 89 human infections with one death.7 Any one of these subtypes could mutate into a virus capable of causing the next human influenza pandemic, but H5N1 is the most dangerous.
History of Pandemic Influenza
Influenza A viruses are known to cause seasonal epidemics as they mutate in small but significant ways from year to year (i.e., antigenic drift). However, worldwide outbreaks or pandemics occur only with the sudden emergence of a radically different virus (i.e., antigenic shift). Influenza viruses are classified on the basis of their 16 hemagglutinin (HA) and nine neuraminidase (NA) subtypes. Pandemic viruses, those with variant HA and NA recombinations, are capable of attacking immunologically naïve populations with devastating results.
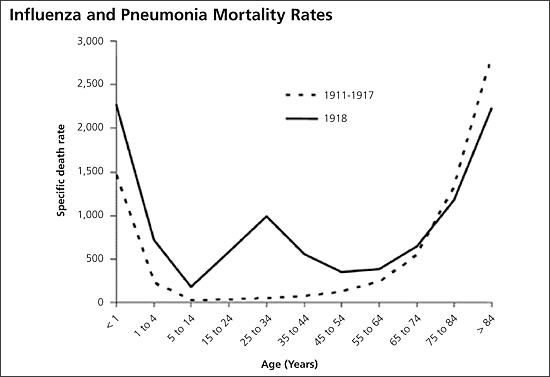
The best example of pandemic influenza is the 1918–1919 “Spanish flu” outbreak of H1N1 influenza, which killed 40 to 100 million persons. The 1918 virus recently has been resurrected, and genetic analysis has confirmed that it is a strain of avian influenza. However, unlike H5N1 (thus far), H1N1 developed the ability to readily infect humans and spread by person-to-person contact.7 Unlike previous pandemics, which had a U-shaped mortality curve (i.e., most deaths in the very young and very old), the 1918 virus killed large numbers of healthy young adults, producing a W-shaped mortality curve (Figure 38,9).10 Since 1918, there have been two other pandemics: the H2N2 “Asian flu” of 1957, which killed more than 2 million persons, and the H3N2 “Hong Kong flu” of 1968, which killed 1 million persons worldwide.4
In 1976, the “swine flu” (also H1N1) provoked a pandemic scare and mass vaccination program, but for unknown reasons, the threat never materialized.11 Although criticized as a failure at the time, the public health response would have been considered heroic had a pandemic emerged. Governments around the world face similar degrees of uncertainty as they confront avian influenza today.
Many public health experts think that another pandemic is overdue. Although the theory of a predictable pandemic cycle has been discarded, it is inevitable that another pandemic will occur.12
Clinical Presentation and Diagnosis
Avian influenza is characterized as a febrile respiratory illness (i.e., fever of at least 100.4° F [38° C]) with leukopenia or lymphopenia, nearly always followed by viral pneumonia with escalating respiratory distress. Symptom onset is usually two to five days after exposure, longer than with human influenza. Chest radiography detects infiltrates a median of seven days after the onset of fever.13 Ventilatory support often is required within 48 hours of hospitalization for acute respiratory distress syndrome. Watery diarrhea, sometimes preceding respiratory symptoms, is fairly common, suggesting viral replication in the intestinal tract.14 Diagnosis is made by viral isolation or detection of H5-specific RNA. Pharyngeal swabs are preferred for diagnosis over nasal swabs because avian viral titers are greater in the throat and lower respiratory tract. Reverse transcriptase-polymerase chain reaction (PCR) assays are more sensitive in detecting H5N1 than commercial rapid antigen tests.14 In February 2006, the U.S. Food and Drug Administration approved the limited release of influenza H/A5 (Asian lineage) Virus Real-Time Reverse Transcription-PCR Primer and Probe Set for more than 140 laboratories in 50 states. This test returns preliminary results within four hours.15
A history of exposure to infected birds is expected because sustained human-to-human transmission is not yet known to occur. Cases of human transmission have occurred after intense exposure from caring for infected persons, but the virus is not yet well enough adapted to humans to be transmitted efficiently from the caregiver to other contacts.16
An unusual feature of H5N1 influenza is that its high mortality rate appears to be related to the production of a “cytokine storm” by an overzealous immune reaction, not necessarily the virus itself.17 However, there is growing evidence that mild cases of H5N1 infection may be more common than previously thought, and that the virus may be adapting to humans. Persons with mild illness do not become ill enough to seek medical attention, so the actual mortality rate may be significantly lower than the 50 percent rate reported in the literature.18
Precautions
Avian influenza is spread through feces and secretions of infected birds. Human cases in Asia have been associated with the consumption of raw duck blood, “mouth-to-beak” resuscitation at cockfights, and cleaning up after or handling infected birds. Exposure to fertilizer containing bird feces also poses a risk. Travelers to Asia are advised to avoid live animal markets and poultry farms. Eating cooked poultry poses no risk, although persons who prepare the uncooked birds could become infected. With the globalization of H5N1, these precautions now apply to Europe and Africa, as well as Asia.
Pandemic Preparedness
Unlike most pandemics, which emerge randomly, H5N1 has been recognized as a likely pandemic candidate for almost 10 years. Experts from the World Health Organization consider the current pandemic alert level to be stage 3 (Table 119), but serious preparations such as antiviral stockpiling and the streamlining of vaccine production started only recently.3 Pandemic preparedness entails pharmacologic and nonpharmacologic interventions.
| Period | Stage | Description |
|---|---|---|
| Interpandemic | 1 | No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may be present in animals. If present in animals, the risk of human infection or disease is considered low. |
| 2 | No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. | |
| Pandemic alert | 3 | Human infection with a new subtype of influenza occurs, but human-to-human transmission has not been reported (or at most, rare instances of spread to a close contact). |
| 4 | Small clusters of infection with limited human-to-human transmission occur, but the spread of the disease is highly localized, suggesting that the virus is not well adapted to humans. | |
| 5 | Larger clusters of influenza infection in humans occur, but human-to-human spread is still localized, suggesting that the virus is becoming better adapted to humans but may not yet be fully transmissible. Characteristics in this phase pose a substantial pandemic risk. | |
| Pandemic | 6 | Increased and sustained transmission of the influenza virus occurs in the general population. |
PHARMACOLOGIC INTERVENTIONS
Pharmacologic approaches include stockpiling antiviral drugs and distributing drugs and vaccines in a timely manner. A multi-tier approach to prioritize vaccine administration is planned (Table 2).20 Although an experimental vaccine for H5N1 is undergoing human testing, it is unlikely to be available to any but a small fraction of persons needing it until late in the pandemic. At least two doses of inactivated H5N1 vaccine likely will be needed; for this reason there is interest in live, attenuated, cold-adapted vaccines, which would provide a more robust immune response but carry an additional risk of viral recombination.4,21 Alternatively, the vaccine supply could be extended by the use of vaccine adjuvants or through intradermal rather than intramuscular administration.1
| Tier | Description | |
|---|---|---|
| 1A | Health care workers with direct patient contact and critical health care support staff | |
| Vaccine and antiviral manufacturing personnel | ||
| 1B | Highest-risk groups: | |
| Patients 65 years and older with at least one high-risk condition | ||
| Patients six months to 64 years of age with at least two high-risk conditions | ||
| Patients hospitalized in the past year because of pneumonia, influenza, or another high-risk condition | ||
| 1C | Household contacts and out-of-home caregivers of children younger than six months | |
| Household contacts and out-of-home caregivers of severely immunocompromised persons | ||
| Pregnant women | ||
| 1D | Key government leaders and critical pandemic public health responders | |
| 2A | Other high-risk groups: | |
| Patients 65 years and older with no high-risk conditions | ||
| Patients six months to 64 years of age with one high-risk condition | ||
| Children six to 23 months of age | ||
| 2B | Other public health emergency responders, public safety workers, utility workers, critical transportation workers, and telecommunications workers | |
| 3 | Other key government health care decision makers | |
| Persons providing mortuary services | ||
| 4 | Healthy persons two to 64 years of age with no high-risk conditions | |
Antiviral medication will provide a vital first response until vaccine becomes available. Drug resistance to amantadine (Symmetrel) and rimantadine (Flumadine) has been reported, especially in viral isolates from Thailand, Vietnam, and Cambodia.22 This resistance may drastically limit the effectiveness of these drugs during a pandemic. The neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza), which inhibit viral release from cells, usually are effective, but only if these agents are used during the first 48 hours of illness. Oseltamivir at a dosage of 75 mg twice daily for five days (for treatment) or 75 mg daily for seven to 10 days (for post-exposure prophylaxis) is the only neuraminidase inhibitor that may be taken orally; thus, it is practical but costly to stockpile.23 Oseltamivir reduces the severity of illness by 40 percent (with reduction of lower respiratory symptoms) and its duration by 30 percent.24 Oseltamivir at lower dosages is indicated in children 12 months and older.
Oseltamivir resistance in H5N1 infections has been reported occasionally.25 It is possible that a mutant strain could be resistant to neuraminidase inhibitors, effectively negating this approach. However, even if the virus remains sensitive, these drugs would have to be distributed and available for use within the first one or two days of illness.
The production of oseltamivir is limited by the availability of an essential precursor, shikimic acid, which is derived from the seed of star anise; all available supplies are being used.26 Little if any oseltamivir will be available in developing countries—where an avian influenza epidemic is most likely to originate—unless it is donated from another country’s stockpile.
Another pharmacologic approach involves calming the immune system’s cytokine storm that results in respiratory failure. The use of statin drugs for hyperlipidemia seems to increase survival rates in patients with septic shock, and there is evidence that these agents also could reduce the risk of dying from pneumonia by 26 percent in patients with H5N1 infection if they are taken in advance of infection.27 It is not yet clear if statins would be effective after the onset of illness, and their use remains controversial. Unlike oseltamivir, statins could be stockpiled and made widely available at a reasonable cost.
NONPHARMACOLOGIC INTERVENTIONS
Nonpharmacologic interventions include the use of influenza surveillance, social distancing (e.g., school closures), travel restrictions, quarantine, surgical and N95 particulate respirator masks, communications networking, and international teamwork to cordon off infected areas. No controlled studies have assessed the effectiveness of masks in preventing influenza transmission, but surgical masks likely will reduce only large-droplet exposure.14,28 Caching portable ventilators in advance and expanding emergency capacity likely will save many lives.29 The severe acute respiratory syndrome epidemic of 2003 provided an unprecedented practice run for these interventions; however, an influenza pandemic would be more difficult to control because of the virus’s much higher reproduction rate (i.e., “R value,” or the number of secondary cases generated by one infected person in a susceptible population).30
Final Comments
Although we are much more prepared for a pandemic than in 1918, this applies far more to developed countries than to the developing continents of Asia and Africa, where many persons live in crowded, unhygienic conditions. Much of the world’s population today constitutes what has been termed a “tinderbox” awaiting the flame of infection.31 Many impoverished nations cannot meet routine public health needs, let alone cope with a pandemic.
Because of budgetary cutbacks, the public health infrastructure in America is weaker than it was during the 1976 swine influenza scare. The number of available hospital beds has been reduced by managed care, and respirator availability and emergency response capacity also are reduced.29 There is little excess capacity left in the country’s health care system, and few places to go if a pandemic hits.
The recent governmental planning report5 on avian influenza provides general guidance but essentially delegates responsibility for preparedness to the state and municipal levels. In addition, the production of influenza vaccine remains a cumbersome six-month process based on the availability of fertile chicken eggs, and it is slowed down by quality-control and manufacturing problems, such as those that occurred during the 2005–2006 influenza season. More governmental support of the vaccine production system will be needed to speed and boost production, possibly through techniques other than egg-based technology. Governmental support also will be required to institute a centralized distribution and rationing system capable of functioning under pandemic conditions.
Table 3 lists Internet resources where additional information about avian and pandemic influenza may be found.
| Centers for Disease Control and Prevention |
| Avian influenza fact sheet (http://www.cdc.gov/flu/avian/gen-info/facts.htm) |
| Emerging Infectious Diseases |
| Avian influenza edition (January 2006;http://www.cdc.gov/ncidod/eid/vol12no01/contents_v12n01.htm) |
| National Academy of Sciences |
| Pandemic influenza workshop summary (http://www.nap.edu/catalog/11150.html) |
| World Health Organization |
| Avian influenza fact sheet (http://www.who.int/csr/don) |
| Infection-control guidelines for health care facilities (http://www.who.int/csr/disease/avian_influenza/guidelines/infectioncontrol1/en/index.html) |
| Threat assessment (http://www.who.int/csr/disease/influenza/H5N1-9reduit.pdf) |
| U.S. Department of Health and Human Services |
| Official government Web site for information on pandemic and avian influenza (http://www.pandemicflu.gov) |