
Am Fam Physician. 2007;76(5):667-671
Patient information: See related handout on actinic keratoses, written by the authors of this article.
Author disclosure: Nothing to disclose.
Actinic keratoses are rough, scaly lesions that commonly occur on sun-exposed areas of the skin. The prevalence of the condition increases with age. Actinic keratoses are thought to be carcinomas in situ, which can progress to squamous cell carcinomas. The decision to treat can be based on cosmetic reasons; symptom relief; or, most importantly, the prevention of malignancy and metastasis. Treatment options include ablative (destructive) therapies such as cryosurgery, curettage with electrosurgery, and photodynamic therapy. Topical therapies are used in patients with multiple lesions. Fluorouracil has been the traditional topical treatment for actinic keratoses, although imiquimod 5% cream and diclofenac 3% gel are effective alternative therapies. There are too few controlled trials comparing treatment modalities for physicians to make sound, evidence-based treatment decisions.
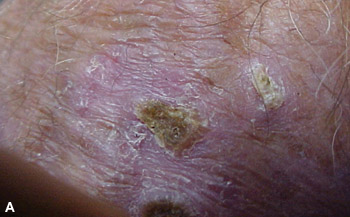
Actinic keratoses are lesions that begin in the epidermis on sun-exposed areas of the body. The lesions appear as rough, scaly patches that range in color from normal skin tone to reddish brown (Figures 1A and 1B). They are often circumscribed and are usually 1 mm to 2.5 cm in diameter, but they can be larger. Patients may present with a single, well-defined lesion or multiple, less-defined lesions covering a large area of skin. Several types of lesions may be present. Most lesions are asymptomatic, but some cause pruritus or a burning sensation.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Actinic keratoses should be treated because of their potential to progress to squamous cell carcinomas. | C | 8–11 |
| Cryosurgery is an effective modality for treating solitary or multiple actinic keratoses. | C | 16–18 |
| Curettage is an effective treatment for patients with a limited number of actinic keratoses, particularly thick, hyperkeratotic lesions. | C | 20 |
| Topical treatment should be considered for patients with multiple lesions. | C | 16 |
Prevalence
Fair-skinned, blue-eyed persons living in sunny climates are most likely to develop actinic keratoses. The lesions take years to develop and usually occur in older persons; they are more common in men than in women.1 Persons with many years of extensive sun exposure are at the greatest risk. More than 80 percent of actinic keratoses occur on areas of the skin with the most sun exposure such as on the head, neck, forearms, and hands.2–4
Risk of Cancer
Actinic keratoses, the most common pre-malignant lesions seen by dermatologists, have the potential to progress to squamous cell carcinomas.8–11 Although most actinic keratoses do not progress to cancer, and as many as 26 percent regress spontaneously,11 up to 60 percent of cutaneous squamous cell carcinomas arise from actinic keratoses.10
Estimates on the risk of actinic keratoses progressing to squamous cell carcinomas vary widely. A prospective, longitudinal study found the risk of progression to be 0.24 percent per year for each actinic keratosis.11 Because most patients have more than one actinic keratosis, another study used these data to determine that the risk of progression to squamous cell carcinoma is 6.1 to 10.2 percent over 10 years per person.12 Two additional studies show that keratoses are associated with squamous cell carcinomas 72 to 97 percent of the time.8,9
Treatment
Actinic keratoses may be treated for cosmetic reasons or for relief of associated symptoms, but the most compelling reason for treatment is to prevent squamous cell carcinomas. Treatment options include ablative (destructive) therapies or topical therapies in patients with multiple lesions.
CRYOSURGERY
Cryosurgery using liquid nitrogen is the most common modality for treating actinic keratoses, although compressed nitrous oxide or carbon dioxide is also used. Liquid nitrogen is sprayed directly on the lesions or applied using a cotton-tipped swab.
Cryosurgery is easily performed in the office setting, produces excellent cosmetic results, and is well tolerated. Potential adverse effects include infection, hypo- or hyperpigmentation, scarring, and hair loss; however, serious reactions are rare. Cryosurgery is best for treating thin, well-demarcated lesions and can be used to treat solitary lesions or small numbers of scattered lesions. Hyperkeratotic lesions are more resistant to cryosurgery and should be debrided before treatment.16,19
CURETTAGE
Curettage, which involves mechanically scraping away abnormal tissue using a sharp curette, is a highly effective modality for treating actinic keratoses. The procedure provides tissue for histologic evaluation but requires local anesthesia.20 Curettage is particularly useful for treating a limited number of actinic keratoses, especially thick, hyperkeratotic lesions. After curettage, electrosurgery may be used to destroy any remaining abnormal tissue and to provide hemostasis. Possible complications include infection, scarring, and hypo- or hyperpigmentation.
PHOTODYNAMIC THERAPY
Photodynamic therapy involves applying a photosensitizing agent to each actinic keratosis, followed by exposure to light of a specific wavelength; this leads to cell death.21 Protocols for using photodynamic therapy to treat actinic keratoses vary with regard to the photosensitizing agent; amount of application; and light source, intensity, and dose. Two protocols are approved for use in the United States.22
The use of the photosensitizing agent aminolevulinic acid (Levulan Kerastick) followed by blue light exposure was approved by the U.S. Food and Drug Administration (FDA) in 1999 for the treatment of nonhyperkeratotic lesions on the face and scalp. The protocol specifies a 14- to 18-hour incubation period between application of aminolevulinic acid and light exposure; however, a subsequent study has demonstrated the effectiveness of shorter incubation periods.23 Another protocol using the photosensitizing agent methyl aminolevulinate (Metvixia; not yet available in the United States) followed by red light exposure was approved by the FDA in 2004. This protocol specifies a three-hour incubation period.
Photodynamic therapy is well tolerated, has excellent cosmetic results, and has reported cure rates between 69 and 93 percent.16,21,24 Potential adverse effects include initial erythema; edema; a burning sensation; pain; and crusting followed by hypo- or hyperpigmentation, ulceration, or scaling.16,21
TOPICAL THERAPIES
Several topical therapies are available for the treatment of actinic keratoses, including various fluorouracil formulations, imiquimod 5% cream (Aldara), and diclofenac 3% gel (Solaraze). Although other topical agents (e.g., colchicine [topical formulation not available in the United States], tretinoin [Retin-A]) are used, there are no comparative phase III studies of these agents.25 Topical therapies are useful for patients with more than 15 actinic keratoses. The anatomic location of the lesions impacts the response time to topical treatments. Actinic keratoses on the face respond the quickest (more quickly than those on the scalp), whereas lesions on the arms usually take the longest to respond.26 After topical treatment, actinic keratoses may reoccur on the treated area.26,27
Fluorouracil
Topical fluorouracil is an established treatment for actinic keratoses and is the standard to which other topical treatments are compared. Fluorouracil cream is available in 5% (Efudex), 1% (Fluoroplex), and 0.5% (Carac) formulations.
Fluorouracil 5% cream is administered twice daily for two to four weeks. The application is associated with local irritation presenting as dryness, erythema, erosion, pain, or edema. Facial irritation and disfigurement associated with fluorouracil 5% cream makes the therapy undesirable to many patients. Studies have evaluated whether intermittent “pulse” applications decrease the adverse effects. However, these studies had small sample sizes and produced inconclusive results.28,29
Fluorouracil 0.5% cream can be used as a neoadjuvant therapy before cryosurgery. A one-week course of fluorouracil 0.5% has been shown to reduce the number of lesions before cryosurgery and to decrease the risk of reoccurrence.32
Imiquimod
Imiquimod 5% cream is also approved for treatment of actinic keratoses. Imiquimod is applied once daily, two or three days a week, for 16 weeks. Several randomized, double-blind, vehicle-controlled trials showed that imiquimod 5% cream produced a complete response in 45 to 57 percent of patients and a partial response (i.e., 75 percent reduction in actinic keratoses) in 59 to 72 percent of patients.33–35 One study showed that in the imiquimod treatment group, 20 percent of participants developed new lesions and none developed squamous cell carcinoma after 24 months of follow-up.27 By comparison, in the vehicle group, 90 percent of participants developed new lesions and one developed squamous cell carcinoma after one year of follow-up.27
Diclofenac
A randomized, double-blind, vehicle-controlled study compared topical diclofenac 3% in hyaluronan 2.5% gel with a hyaluronan 2.5% vehicle.38 Participants applied the treatments twice daily for 90 days, with follow-up 30 days after the end of treatment. Approximately 50 percent of participants in the treatment group had complete resolution compared with 20 percent in the vehicle group.38
| Trials* | Treatment regimen | No. of participants | Complete response (%) | Partial response†(%) |
|---|---|---|---|---|
| Diclofenac 3% (Solaraze) in hyaluronan 2.5% gel | ||||
| Wolf, et al., 200138 | Twice daily for 90 days | 120 | Treatment: 47.0 | — |
| Vehicle: 19.0 | ||||
| Imiquimod 5% cream (Aldara) | ||||
| Korman, et al., 200535 | Once daily, three times per week, for 16 weeks | 492 | Treatment: 48.3 | Treatment: 64.0 |
| Vehicle: 7.2 | Vehicle: 13.6 | |||
| Lebwohl, et al., 200433 | Once daily, two times per week, for 16 weeks | 436 | Treatment: 45.1 | Treatment: 59.1 |
| Vehicle: 3.2 | Vehicle: 11.8 | |||
| (95% CI, 34.9 to 49)‡ | (95% CI, 39.5 to 55.1)‡ | |||
| Szeimies, et al., 200434 | Once daily, three times per week, for 16 weeks | 286 | Treatment: 57.1 | Treatment: 72.1 |
| Vehicle: 2.2 | Vehicle: 4.3 | |||
Chemical peels
Facial peels using Jessner's solution (i.e., resorcinol, lactic acid, and salicylic acid in ethanol) and trichloroacetic acid 35% (Tri-Chlor) are comparable with fluorouracil in reducing actinic keratoses and reccurrence.39 Patients may prefer a chemical peel over fluorouracil because of the convenience of a single application.