
Am Fam Physician. 2010;81(4):477-484
Author disclosure: Nothing to disclose.
Preventing preterm delivery remains one of the great challenges in modern medicine. Preterm birth rates continue to increase and accounted for 12.7 percent of all U.S. births in 2005. The etiology of preterm delivery is unclear, but is likely to be complex and influenced by genetics and environmental factors. Women with previous preterm birth are at increased risk of subsequent preterm delivery and may be candidates for treatment with antenatal progesterone. Fetal fibronectin testing and endovaginal ultrasonography for cervical length are useful for triage. For the patient in preterm labor, only antenatal corticosteroids and delivery in a facility with a level III neonatal intensive care unit have been shown to improve outcomes consistently. Tocolytic agents may delay delivery for up to 48 hours, enabling the administration of antenatal corticosteroids or maternal transfer. Routine use of antibiotics in preterm labor is not indicated except for group B streptococcus prophylaxis or treatment of chorioamnionitis.
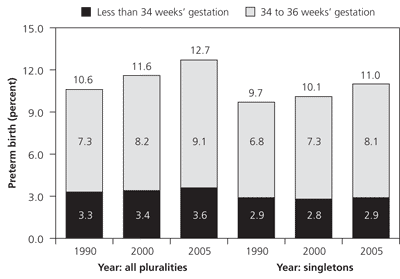
In 2005, 12.7 percent of American births were preterm, defined as occurring before 37 weeks' gestation.1 The rate of preterm delivery has increased 20 percent since 1990; recent increases have been associated primarily with late preterm (i.e., 34 to 36 weeks' gestation) deliveries (Figure 1).1 Rates of preterm delivery differ among socioeconomic groups and races. For example, black women had an 18.4 percent rate of preterm delivery in 2005 compared with non-Hispanic white women, who had a rate of 11.7 percent, and Hispanic women, who had a rate of 12.1 percent.1 Increased rates of preterm delivery are also noted among demographic groups delaying pregnancy and those using assisted reproductive technology.2 About one half of preterm deliveries are the result of spontaneous labor with intact membranes, one fourth are associated with preterm premature rupture of membranes (PPROM), and one fourth are iatrogenic.3 Iatrogenic factors include elective preterm delivery by induction of labor or cesarean delivery for medical indications, such as hypertensive diseases of pregnancy, intra-uterine growth restriction, placental abruption, or nonreassuring fetal surveillance.4 This review focuses primarily on spontaneous preterm labor with intact membranes.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Antenatal progesterone supplementation decreases the incidence of preterm delivery in high-risk patients. | A | 28, 63 |
| Administration of corticosteroids in women with preterm labor between 26 and 34 weeks' gestation reduces the incidence of neonatal death, respiratory distress syndrome, and intraventricular hemorrhage. | A | 44, 45 |
| Corticosteroids are effective in women with premature labor with intact membranes, preterm premature rupture of membranes, and hypertensive disorders of pregnancy. | ||
| If delivery is not imminent, maternal transfer to a facility with a level III neonatal intensive care unit is indicated. | C | 50, 59 |
| In symptomatic patients, calcium channel blockers reduce the risk of delivery within seven days and before 34 weeks' gestation. Calcium channel blockers are better tolerated than beta mimetics. | A | 57 |
| In symptomatic patients, beta mimetics decrease the risk of delivery within 48 hours. They are associated with significant adverse effects. | A | 58 |
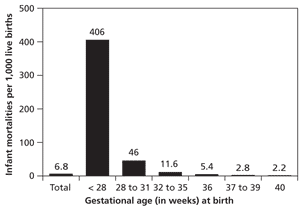
Despite improvements in neonatal care, infants born prematurely have increased rates of mortality, and those who survive may have neurodevelopmental and medical disabilities.5 Infant mortality rates are a function of gestational age at birth and increase significantly before 32 weeks' gestation (Figure 2).5 Although neonatal morbidity and mortality increase at earlier gestational ages, the high proportion of preterm deliveries at 34 to 36 weeks' gestation results in the majority of admissions to neonatal intensive care units (NICUs), primarily from respiratory complications.
Risk Factors for Preterm Delivery
Table 1 lists risk factors for preterm delivery.6 An interpregnancy interval of less than six months increases the risk of preterm delivery (odds ratio = 2.2; 95% confidence interval [CI], 1.3 to 3.6).7 A history of previous preterm birth is the most important historical risk factor for subsequent preterm delivery, with a relative risk (RR) of 2.5 (95% CI, 1.9 to 3.2).8 A shorter penultimate (previous) pregnancy or a history of more than one preterm birth confer an even greater likelihood of subsequent preterm delivery.9
| Maternal characteristics |
| Black race |
| Interpregnancy interval of less than six months |
| Physically strenuous or stressful work |
| Prepregnancy body mass index = 19 kg per m2 |
| Pregnancy history |
| Previous preterm delivery |
| Pregnancy characteristics |
| Bacterial vaginosis, Chlamydia infection |
| Cocaine or heroin use |
| History of cervical cone biopsy or loop electrosurgical excision procedure |
| Intrauterine infection |
| Maternal abdominal surgery |
| Maternal medical disorders such as thyroid disease, diabetes mellitus, or hypertension |
| Multiple gestation |
| Nongenital tract infection (asymptomatic bacteriuria, pneumonia, appendicitis) |
| Periodontal disease |
| Polyhydramnios or oligohydramnios |
| Shortened cervix (< 3.0 cm) |
| Tobacco use |
| Uterine anomalies |
| Vaginal bleeding caused by placental abruption or placenta previa |
A history of cervical conization or loop electrosurgical excision procedure of the cervical transformation zone increases the risk of preterm delivery (RR = 1.99; 95% CI, 1.81 to 2.20).10 Tobacco use is moderately associated with preterm birth (RR = 1.2 to 1.6).11 Smoking is also associated with higher rates of recurrent preterm birth.6 Infection is one of the primary biologic pathways leading to preterm labor.6 Bacterial vaginosis (BV; RR = 1.5 to 3.0) and genitourinary infection with Chlamydia (RR = 2.2; 95% CI, 1.03 to 4.78) are associated with preterm delivery.12 Maternal periodontal infection also increases the risk of preterm delivery (RR = 1.6; 95% CI, 1.1 to 2.3).13
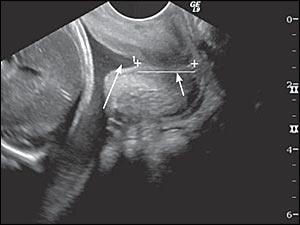
A shortened cervix (i.e., generally less than 3.0 cm) or a funneling configuration at the internal os observed in second trimester transvaginal ultrasonography (Figure 3) increases the likelihood of preterm delivery.14,15 Measurements before 14 weeks' gestation lack predictive value. Cervical effacement begins in normal pregnancies at about 32 weeks' gestation, limiting the usefulness of measurement in asymptomatic patients after 32 weeks' gestation.16 Table 2 lists predictive values of cervical length for preterm delivery.14 A shorter cervix confers a higher risk of preterm delivery.14
Prevention of Preterm Delivery
Smoking cessation interventions decrease the RR for delivering infants with low birth weight (RR = 0.81; 95% CI, 0.70 to 0.94), and for preterm birth (RR = 0.84; 95% CI, 0.72 to 0.98).17
Screening and treatment of BV for the prevention of preterm delivery remains controversial. In patients with a history of preterm birth, screening for and treatment of BV result in a decreased rate of PPROM and low–birth-weight newborns without affecting the incidence of preterm birth.20 When asymptomatic, low-risk women were screened early in the second trimester and infected patients treated with clindamycin (Cleocin, administered vaginally in two studies and orally in one), rates of preterm delivery and second trimester miscarriage were reduced.21–24 However, the U.S. Preventive Services Task Force recommends against screening low-risk patients for BV and rates the evidence as insufficient to recommend for or against screening and treatment of high-risk patients.25 Routine screening and treatment of BV in low-risk patients should wait until best practices for timing and methods of screening, as well as treatment of infection, have been established.
Although most studies used Gram stain for the diagnosis of BV, most physicians use the Amsel criteria. For a BV diagnosis using the Amsel criteria, at least three of the following factors must be present: amine odor with potassium hydroxide; clue cells on normal saline wet mount; pH greater than 4.5; and a thin, homogenous vaginal discharge.26
Centers for Disease Control and Prevention suggestions for the treatment of BV in pregnancy include oral clindamycin at a dosage of 300 mg twice daily for seven days, metronidazole (Flagyl) at a dosage of 500 mg twice daily for seven days, or metronidazole at a dosage of 250 mg three times daily for seven days.27 Patients should be reevaluated one month after treatment.27 Clindamycin is preferred.
Antenatal progesterone prevents the formation of cellular gap junctions and maintains uterine quiescence. A meta-analysis of randomized controlled trials involving women at an increased risk of preterm delivery showed that progesterone therapy decreased the risk of delivery before 37 weeks' gestation (RR = 0.57; 95% CI, 0.36 to 0.90; number needed to treat = 6) without an increase in fetal congenital anomalies.28 One of the trials included in the analysis used 17-alpha hydroxyprogesterone caproate (250 mg intramuscularly weekly from 16 to 20 weeks' gestation through 36 weeks' gestation),29 whereas another trial used natural progesterone suppositories (100 mg vaginally daily from 24 to 34 weeks' gestation).30 Overall, the analysis found that the trials' effect on neonatal mortality was uncertain,28 although 17-alpha hydroxyprogesterone injections resulted in lower rates of necrotizing enterocolitis and intraventricular hemorrhage, and less need for supplemental oxygen.29 Prophylactic vaginal progesterone from 24 to 34 weeks' gestation in women with a cervical length of less than 15 mm at 22 weeks' gestation resulted in decreased rates of preterm birth (RR = 0.56; 95% CI, 0.56 to 0.86).31 However, progesterone treatment in twin pregnancies did not improve outcomes.32 The American College of Obstetricians and Gynecologists recommends limiting antenatal progesterone prophylaxis to women with a previous spontaneous birth at less than 37 weeks' gestation.33
Initial Evaluation of the Patient with Preterm Contractions
Only 30 to 60 percent of women presenting with preterm contractions go on to deliver prematurely.34 The three antenatal interventions that have been proven effective in premature labor are transfer to a facility with a NICU, maternal corticosteroid administration, and antibiotic prophylaxis for group B streptococcus (GBS).35 The physician should obtain a thorough history and assess fetal well-being, membrane integrity, and the possibility of underlying infection. Table 3 summarizes the steps involved in the initial assessment of patients with premature contractions.
| Assessment questions | Comments |
|---|---|
| What is the gestational age? | Verify dates by clinical history and ultrasonography |
| Are the membranes ruptured? | History of leaking fluid; observed leakage of fluid from cervical os on sterile speculum examination; nitrazine-positive reaction of fluid; ferning of fluid; ultrasonography for oligohydramnios; amnioinfusion of indigo carmine, if available |
| Is the patient in labor? | Observe for contractions accompanied by cervical effacement and dilation |
| Is there an infection? | Group B streptococcus carrier status; urinary tract infection; bacterial vaginosis; trichomoniasis; gonorrhea or chlamydia |
| What is the likelihood that the patient will deliver prematurely? | Consider endovaginal ultrasonography of cervical length and/or fetal fibronectin testing |
ASSESS FOR RUPTURE OF MEMBRANES
Sterile speculum examination is recommended to assess whether the membranes have ruptured, as well as to collect a sample for fetal fibronectin analysis.36 If pooled fluid is not visible and suspicion remains high for rupture of the fetal membranes, the patient should be reexamined after prolonged recumbency.37 A sample of fluid can be tested for alkalinity using the nitrazine test (accuracy of 97 percent) and ferning (accuracy of 84 to 100 percent).38 False-positive nitrazine results can be obtained in the presence of blood, semen, alkaline urine, and vaginal infections. To evaluate for ferning, a sample of fluid from the vaginal pool is placed on a glass slide and allowed to air dry for 10 minutes. The development of a fern-like crystalline pattern strongly suggests the presence of amniotic fluid. Cervical mucus may also cause ferning and may lead to an incorrect diagnosis of rupture of membranes.38
Oligohydramnios on ultrasonography supports the presence of ruptured membranes, but it is not diagnostic. When there is high suspicion of ruptured membranes despite negative findings on speculum examination, diagnosis may be confirmed with observation of passage of blue fluid through the cervical os after an ultrasound-guided, transabdominal amnioinfusion of indigo carmine (1 mL in 9 mL of normal saline).37 This test may not be available in all institutions.
ASSESS FOR INFECTION
Because bacteriuria and pyelonephritis are associated with preterm delivery, urine should be obtained for microscopic analysis and culture.36 If the patient has not already been screened, a rectovaginal culture should be obtained for determination of GBS-carrier status, and prophylaxis should be initiated, if necessary. Screening for gonorrhea and chlamydia should be considered if not performed previously. Symptomatic patients should be evaluated for BV and trichomoniasis, although treatment of these infections will not affect pregnancy duration.25
DETERMINE THE LIKELIHOOD OF TRUE LABOR
Labor is defined as regular uterine contractions that are accompanied by descent of the presenting fetal part, and progressive dilation and effacement of the cervix. Accuracy of clinical diagnosis is increased when there are at least six contractions per hour, the cervix is dilated to at least 3.0 cm, effacement is at least 80 percent, membranes are ruptured, or there is vaginal bleeding.35 However, patients may present with an incomplete picture. Fetal fibronectin testing and cervical ultrasonography help determine the likelihood of preterm delivery.
Fetal Fibronectin Testing. Oncofetal fibronectin is a placental glycoprotein that is thought to play a role in implantation and maintenance of choriodecidual attachment throughout pregnancy. The fetal fibronectin assay is superior to cervical dilatation and contraction frequency in predicting preterm delivery in symptomatic women.39 The negative predictive value of fetal fibronectin testing is greater than 99 percent for delivery within 14 days.40 The positive predictive value is 13 to 30 percent for delivery within seven to 10 days.40 The test should not be done if the patient had a vaginal examination, sexual intercourse, or endovaginal ultrasonography within the previous 24 hours.40 Other confounders to fetal fibronectin testing include vaginal bleeding, rupture of membranes, abnormal vaginal flora, and use of vaginal lubricants or disinfectants.41
Cervical Ultrasonography. As with the fetal fibronectin testing, cervical ultrasonography is more useful in ruling out than diagnosing preterm labor. Decreased cervical length is associated with increased risk of subsequent delivery among patients presenting with premature contractions.15 Patients with a cervical length of at least 3.0 cm are unlikely to deliver within seven days (1 percent in one study).42 However, it is more problematic establishing a cervical length that is usefully predictive of eventual preterm birth.15 In one study, most deliveries within seven days occurred when the cervical length was less than 1.5 cm.43
Fetal Fibronectin Testing and Cervical Ultrasonography. The use of combined cervical ultrasonography and fetal fibronectin testing is more accurate than the use of either test alone.42 In one study of 215 symptomatic patients, 23.1 percent of those with a cervical length less than 3.0 cm delivered within seven days.42 Within that same group, 11.4 percent delivered when the fetal fibronectin test was negative, and 44.7 percent delivered when it was positive.
Management of Preterm Labor
Management of preterm labor consists of corticosteroids to improve fetal outcomes, antibiotics for prophylaxis of GBS infection, and limited tocolysis.
ANTENATAL CORTICOSTEROID THERAPY FOR FETAL MATURATION
When administered to women with preterm labor between 24 and 34 weeks' gestation, betamethasone (two 12-mg intramuscular doses 24 hours apart) or dexamethasone (6 mg intramuscularly every 12 hours for four doses) results in a decreased incidence of neonatal mortality, respiratory distress syndrome, and intra-ventricular hemorrhage.44 Antenatal corticosteroids also benefit patients with PPROM and those with hypertensive syndromes. Repeat courses of corticosteroids are not recommended.45
PERINATAL GBS PROPHYLAXIS
GBS prophylaxis is unnecessary for women with potential preterm delivery who have been screened and are GBS negative.46 For those at less than 37 weeks' gestation who have not yet been screened, a rectovaginal culture or rapid streptococcal test should be obtained, and prophylaxis should be started.47 Full antibiotic prophylaxis is indicated in patients who are culture-positive for GBS, who had GBS bacteriuria prenatally, or who had a prior newborn infected with GBS (Table 4).46 Antibiotics do not affect the outcome of preterm labor in patients with intact membranes, except when given for treatment of suspected infection (e.g., chorioamnionitis) or for GBS prophylaxis.48
| Regimen | Medication | |
|---|---|---|
| Recommended prophylaxis | Penicillin G: initial dose of 5 million units IV, then 2.5 million units IV every four hours until delivery | |
| Alternative prophylaxis | Ampicillin: initial dose of 2 g IV, then 1 g IV every four hours until delivery | |
| For patients who are allergic to penicillin | ||
| Not at high risk of anaphylaxis | Cefazolin: initial dose of 2 g IV, then 1 g IV every eight hours until delivery | |
| At high risk of anaphylaxis and GBS susceptible to clindamycin (Cleocin) and erythromycin | Clindamycin: 900 mg IV every eight hours until delivery | |
| or | ||
| Erythromycin: 500 mg IV every six hours until delivery | ||
| At high risk of anaphylaxis and GBS resistant to clindamycin or erythromycin, or GBS susceptibility unknown | Vancomycin: 1 g every 12 hours until delivery | |
TOCOLYSIS
In contrast with antenatal steroid administration, tocolysis lacks robust outcome-based research support. The use of tocolytics decreases the odds of delivery within 48 hours, but has not consistently been shown to improve neonatal and perinatal outcomes.49 A delay of delivery for 48 hours allows for corticosteroid administration or maternal transfer.50 Table 5 summarizes individual tocolytic dosages, contraindications, and adverse effect information.34,49,51–58 General contraindications to tocolysis include fetal distress, chorioamnionitis, and maternal instability.35
| Medication (class) | Dosage | Comment | Contraindications and adverse effects | References |
|---|---|---|---|---|
| Indomethacin (Indocin; class: NSAIDs) | Loading dose: 50 mg rectally or 50 to 100 mg orally Maintenance dosage: 25 to 50 mg orally every four hours for 48 hours Total 24-hour dose should not be greater than 200 mg | NSAIDs theoretically intervene more proximally in the labor cascade than other agents; effectiveness similar to other agents Maternal adverse effect profile is favorable Other NSAIDs (sulindac [Clinoril], ketorolac [formerly Toradol]) may be used May be optimal choice for tocolysis before 32 weeks' gestation | Contraindications: maternal renal or hepatic impairment, active peptic ulcer disease, oligohydramnios Maternal adverse effects: nausea, heartburn Fetal adverse effects: constriction of the ductus arteriosus (not recommended after 32 weeks' gestation), pulmonary hypertension, reversible decrease in renal function with oligohydramnios, intraventricular hemorrhage, hyperbilirubinemia, necrotizing enterocolitis | 34, 49, 51–54 |
| Magnesium sulfate | 4- to 6-g bolus intravenously over 20 minutes, then 1 to 2 g per hour (3 g per hour maximum) | In widespread use in the United States, although meta-analysis fails to demonstrate improvement in outcomes; comparison studies demonstrate similar effectiveness to other agents in delay of delivery | Contraindication: myasthenia gravis Maternal adverse effects: flushing, lethargy, headache, muscle weakness, diplopia, dry mouth, pulmonary edema, cardiac arrest Newborn adverse effects: lethargy, hypotonia, respiratory depression, demineralization with prolonged use | 34, 52, 53, 55, 56 |
| Nifedipine (Procardia; class: calcium channel blockers) | 30-mg loading dose orally, then 10 to 20 mg every four to six hours | May offer the best outcomes of the tocolytic agents May prolong pregnancy for seven days; delivery after 34 weeks' gestation is also increased Decreased incidence of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice Neonatal mortality not affected | Contraindication: maternal hypotension Maternal adverse effects: flushing, headache, dizziness, nausea, transient hypotension No fetal adverse effects noted | 34, 49, 57 |
| Terbutaline (formerly Brethine; class: beta mimetics) | 0.25 mg subcutaneously every 20 to 30 minutes for four to six doses | Appropriate as the first-line agent Beta mimetics may delay delivery for 48 hours, but neonatal outcomes are variable and maternal adverse effects common | Maternal contraindications: heart disease, poorly controlled diabetes mellitus, thyrotoxicosis Maternal adverse effects: cardiac arrhythmias, pulmonary edema, myocardial ischemia, hypotension, tachycardia, hyperglycemia, hyperinsulinemia, hypokalemia, antidiuresis, altered thyroid function, physiologic tremor, palpitations, nervousness, nausea, vomiting, fever, hallucinations Fetal and newborn adverse effects: tachycardia, hypoglycemia, hypocalcemia, hyperbilirubinemia, hypotension, intraventricular hemorrhage | 34, 49, 52, 58 |
Delivery of the Preterm Infant
The odds of neonatal death in infants weighing less than 1,500 g (3 lb, 5 oz) decrease as the level of NICU care at the birth hospital increases.59 Maternal transfer to a facility with a level III NICU is indicated if delivery is not imminent and if level III NICU services are unavailable at the current facility.50,59 The preterm fetus is more susceptible to injury from acidosis and anoxia, and therefore should have continuous electronic fetal monitoring.34 The immaturity of the fetus and adverse effects of tocolytic medications complicate fetal surveillance.
Malpresentations are more common at earlier gestations and should be anticipated. There is no evidence that prophylactic episiotomy, forceps delivery, or cesarean delivery (other than for nonvertex presentation) improve neonatal outcomes in preterm delivery.60 Retained placenta is more common than with term pregnancies.61 Umbilical cord blood acid-base studies should be considered after delivery.62
