Am Fam Physician. 2010;82(8):901-904
Author disclosure: Nothing to disclose.
Milnacipran (Savella) is a serotonin-norepinephrine reuptake inhibitor labeled for the treatment of fibromyalgia. It is the third medication marketed for this use.1
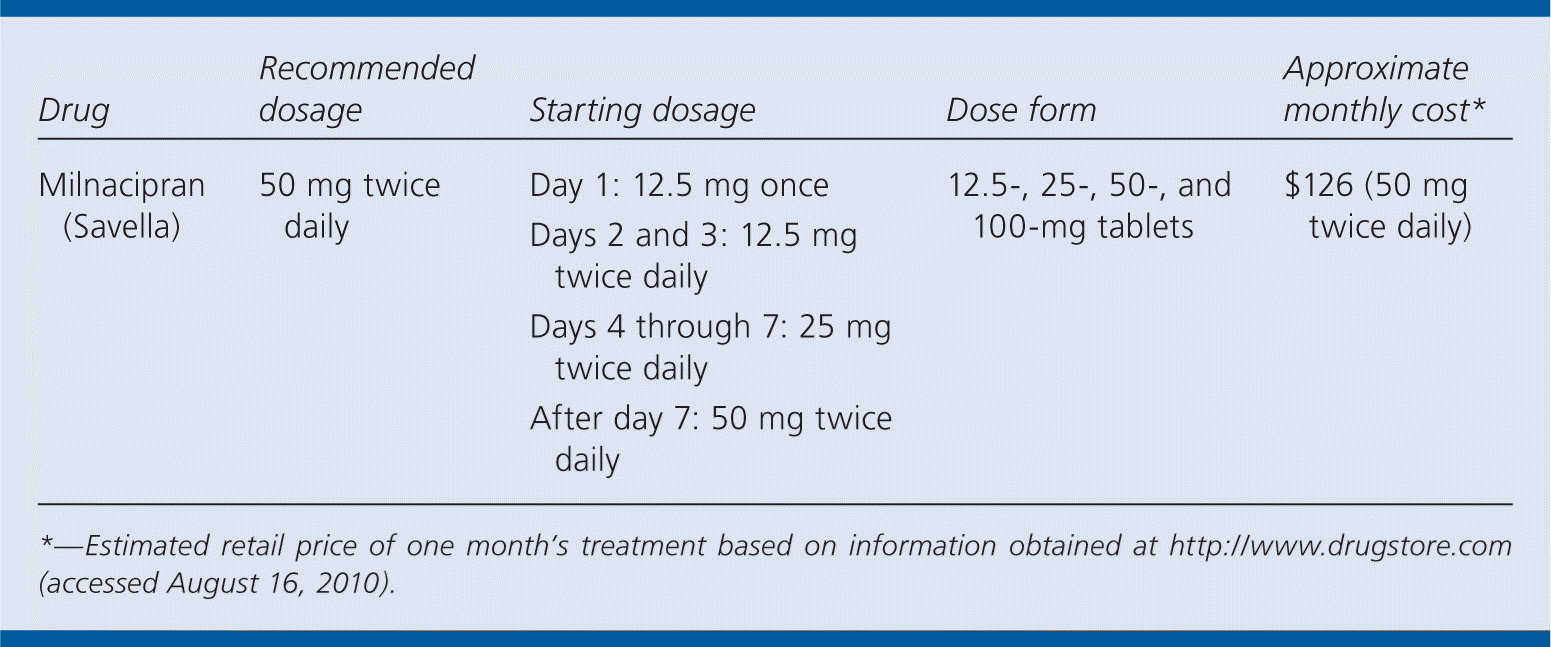
| Drug | Recommended dosage | Starting dosage | Dose form | Approximate monthly cost* |
|---|---|---|---|---|
| Milnacipran (Savella) | 50 mg twice daily | Day 1: 12.5 mg once Days 2 and 3: 12.5 mg twice daily Days 4 through 7: 25 mg twice daily After day 7: 50 mg twice daily | 12.5-, 25-, 50-, and 100-mg tablets | $126 (50 mg twice daily) |
SAFETY
Because milnacipran is a serotonin-norepinephrine reuptake inhibitor, it can cause serotonin syndrome.2 The manufacturer recommends not using milnacipran with monoamine oxidase inhibitors or within 14 days of discontinuing them. Also, monoamine oxidase inhibitors should not be started within five days of discontinuing milnacipran. In patients with narrow-angle glaucoma, use of milnacipran is contraindicated.
Milnacipran carries the same warnings of increased suicidality as antidepressants. It may increase the risk of convulsions in patients with seizure disorders. Hyponatremia, sexual dysfunction, dysuria, and urinary retention, especially in men with preexisting prostatic hypertrophy or prostatitis, may occur with milnacipran. Bleeding disorders are possible in patients using milnacipran, especially in those taking aspirin.3
About one in five patients without hypertension will have a sustained increase in blood pressure (140/90 mm Hg or greater). Among patients with preexisting hypertension, a typical dose will increase systolic blood pressure by more than 15 mm Hg in 7 percent of patients and diastolic blood pressure by more than 10 mm Hg in 8 percent of patients. It increases the heart rate by more than 20 beats per minute in about 8 percent of patients. A similar proportion of patients will report palpitations. Patients who experience a sustained increase in blood pressure or heart rate should receive a lower dose or discontinue treatment. Use of milnacipran has not been evaluated in patients with cardiac rhythm disorders.3
Abrupt discontinuation of milnacipran can induce withdrawal symptoms, including dysphoria and agitation. Patients should receive tapered doses when discontinuing treatment.
Milnacipran is U.S. Food and Drug Administration pregnancy category C. It is not approved for use in children.
TOLERABILITY
More patients will discontinue a typical dose of milnacipran than will discontinue placebo (27 versus 10 percent; number needed to harm = 6). Gastrointestinal symptoms, including nausea (35 percent) and constipation (16 percent), are the most common adverse effects of milnacipran. About 18 percent of patients will report headache.3,4 Milnacipran has a similar adverse effect profile to other medications in its class.1,4–6
EFFECTIVENESS
Milnacipran has been studied in four randomized placebo-controlled trials of patients with fibromyalgia, which is defined as chronic, widespread pain and pain at 11 or more tender points. At three months, about twice as many patients on a typical dose will report overall improvement compared with those receiving placebo (32.8 versus 17.3 percent; P = .003; number needed to treat = 6). Patients who respond continue to experience benefit at six months. Mental health scores and levels of bodily pain and fatigue also improve with treatment. However, measures of impact on daily life (e.g., physical function, work absence, job ability) are not improved.6 Milnacipran has not been studied over the long term. Recent review of published and unpublished data indicated that milnacipran, pregabalin (Lyrica), and duloxetine (Cymbalta) have different effects on the key symptoms of fibromyalgia syndrome and differences in adverse effects and contraindications.7
PRICE
A one-month supply of milnacipran (50 mg twice daily) costs $126. In comparison, a one-month supply of pregabalin (150 mg twice daily) costs $176, and a one-month supply of duloxetine (60 mg daily) costs $154.
SIMPLICITY
The recommended dosage of milnacipran is 50 mg twice daily. Patients should be started at a single dose of 12.5 mg on day 1; 12.5 mg twice daily on days 2 and 3; 25 mg twice daily on days 4 through 7; and 50 mg twice daily after that. In patients who do not respond to this maintenance dosage, the dosage may need to be increased to 100 mg twice daily.3
Bottom Line
Milnacipran can decrease the overall pain scores and improve symptoms of fibromyalgia. Safety in patients with severe comorbid medical and psychiatric conditions is unknown because these groups of patients were excluded from the study.1 Milnacipran can be useful in select patients with fibromyalgia in whom first- and second-line treatments have failed.