
Am Fam Physician. 2011;83(6):719-724
A more recent article on blood product transfusion in adults is available.
Author disclosure: Nothing to disclose.
Red blood cell transfusions are used to treat hemorrhage and to improve oxygen delivery to tissues. Transfusion of red blood cells should be based on the patient's clinical condition. Indications for transfusion include symptomatic anemia (causing shortness of breath, dizziness, congestive heart failure, and decreased exercise tolerance), acute sickle cell crisis, and acute blood loss of more than 30 percent of blood volume. Fresh frozen plasma infusion can be used for reversal of anticoagulant effects. Platelet transfusion is indicated to prevent hemorrhage in patients with thrombocytopenia or platelet function defects. Cryoprecipitate is used in cases of hypofibrinogenemia, which most often occurs in the setting of massive hemorrhage or consumptive coagulopathy. Transfusion-related infections are less common than noninfectious complications. All noninfectious complications of transfusion are classified as noninfectious serious hazards of transfusion. Acute complications occur within minutes to 24 hours of the transfusion, whereas delayed complications may develop days, months, or even years later.
Blood transfusion can be a lifesaving procedure, but it has risks, including infectious and noninfectious complications. There is debate in the medical literature concerning the appropriate use of blood and blood products. Clinical trials investigating their use suggest that waiting to transfuse at lower hemoglobin levels is beneficial.1,2 This review will consider the indications for transfusion of blood and blood products, and will discuss common noninfectious complications associated with transfusion.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| The threshold for transfusion of red blood cells should be a hemoglobin level of 7 g per dL (70 g per L) in adults and most children. | A | 1, 2, 6 | RCTs in adults and children with a critical illness |
| A restrictive transfusion strategy (hemoglobin level of 7 to 9 g per dL [70 to 90 g per L]) should not be used in preterm infants or children with cyanotic heart disease, severe hypoxemia, active blood loss, or hemodynamic instability. | B | 2 | RCT in children with a critical illness |
| Transfusion of plasma should be considered in a patient who has an International Normalized Ratio greater than 1.6 with active bleeding, or in a patient receiving anticoagulant therapy before an invasive procedure. | C | 8 | Consensus conference recommendations |
| Platelets should not be transfused in patients with thrombotic thrombocytopenic purpura or heparin-induced thrombocytopenia unless a life-threatening hemorrhage has occurred. | C | 10, 11 | Guidelines based on case reports |
Red Blood Cells
Packed red blood cells (RBCs) are prepared from whole blood by removing approximately 250 mL of plasma. One unit of packed RBCs should increase levels of hemoglobin by 1 g per dL (10 g per L) and hematocrit by 3 percent. In most areas, packed RBC units are filtered to reduce leukocytes before storage, which limits febrile nonhemolytic transfusion reactions (FNHTRs), and are considered cytomegalovirus safe.3
RBC transfusions are used to treat hemorrhage and to improve oxygen delivery to tissues. Transfusion of RBCs should be based on the patient's clinical condition.4 Indications for RBC transfusion include acute sickle cell crisis (for stroke prevention), or acute blood loss of greater than 1,500 mL or 30 percent of blood volume.4 Patients with symptomatic anemia should be transfused if they cannot function without treating the anemia.4 Symptoms of anemia may include fatigue, weakness, dizziness, reduced exercise tolerance, shortness of breath, changes in mental status, muscle cramps, and angina or severe congestive heart failure. The 10/30 rule—transfusion when a patient has a hemoglobin level less than or equal to 10 g per dL (100 g per L) and a hematocrit level less than or equal to 30 percent—was used until the 1980s as the trigger to transfuse, regardless of the patient's clinical presentation.4,5
In 1999, a randomized, multicenter, controlled clinical trial evaluated a restrictive transfusion trigger (hemoglobin level of 7 to 9 g per dL [70 to 90 g per L]) versus a liberal transfusion trigger (hemoglobin level of 10 to 12 g per dL [100 to 120 g per L]) in patients who were critically ill.1 Restrictive transfusion practices resulted in a 54 percent relative decrease in the number of units transfused and a reduction in the 30-day mortality rate. The authors recommended transfusion when hemoglobin is less than 7 g per dL, and maintenance of a hemoglobin level between 7 to 9 g per dL.1 A recently updated Cochrane review supports the use of restrictive transfusion triggers in patients who do not have cardiac disease.6
A similar study was carried out in critically ill children.2 The restrictive transfusion trigger was a hemoglobin level of 7 g per dL, with a target level of 8.5 to 9.5 g per dL (85 to 95 g per L). The liberal transfusion trigger was a hemoglobin level of 9.5 g per dL, with a target level of 11 to 12 g per dL (110 to 120 g per L). Patients in the restrictive group received 44 percent fewer blood transfusions, with no difference in rates of multiple organ dysfunction syndrome or death. The restrictive transfusion strategy is useful for children who are stable patients in intensive care. It should not be used in preterm neonates or in children with severe hypoxemia, active blood loss, hemodynamic instability, or cyanotic heart disease.2
Plasma
Plasma products available in the United States include fresh frozen plasma and thawed plasma that may be stored at 33.8 to 42.8°F (1 to 6°C) for up to five days. Plasma contains all of the coagulation factors. Fresh frozen plasma infusion can be used for reversal of anticoagulant effects. Thawed plasma has lower levels of factors V and VIII and is not indicated in patients with consumption coagulopathy (diffuse intravascular coagulation).3
Plasma transfusion is recommended in patients with active bleeding and an International Normalized Ratio (INR) greater than 1.6, or before an invasive procedure or surgery if a patient has been anticoagulated.7,8 Plasma is often inappropriately transfused for correction of a high INR when there is no bleeding. Supportive care can decrease high-normal to slightly elevated INRs (1.3 to 1.6) without transfusion of plasma. Table 1 gives indications for plasma transfusion.7–9
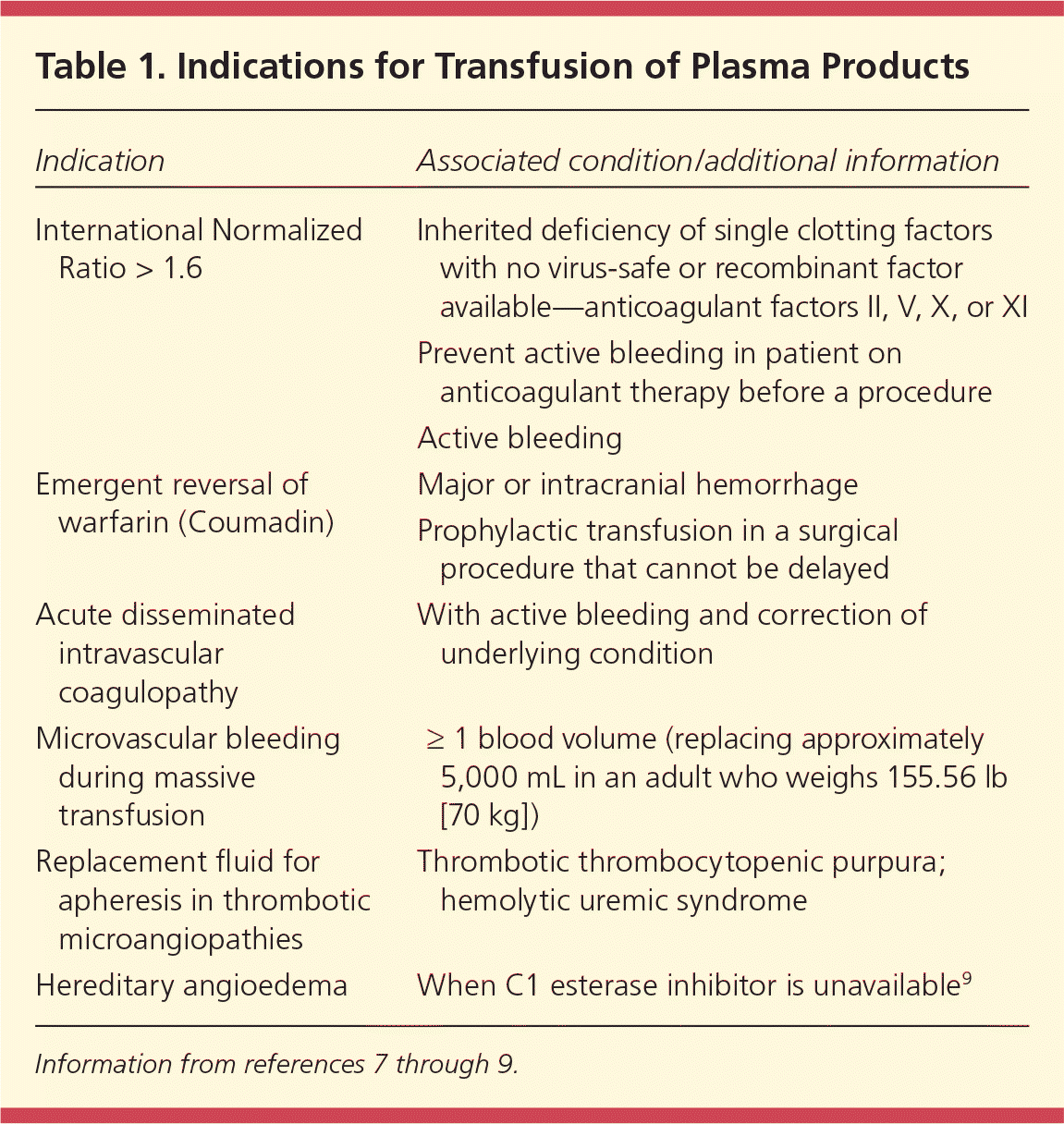
| Indication | Associated condition/additional information |
|---|---|
| International Normalized Ratio > 1.6 | Inherited deficiency of single clotting factors with no virus-safe or recombinant factor available—anticoagulant factors II, V, X, or XI |
| Prevent active bleeding in patient on anticoagulant therapy before a procedure | |
| Active bleeding | |
| Emergent reversal of warfarin (Coumadin) | Major or intracranial hemorrhage |
| Prophylactic transfusion in a surgical procedure that cannot be delayed | |
| Acute disseminated intravascular coagulopathy | With active bleeding and correction of underlying condition |
| Microvascular bleeding during massive transfusion | ≥ 1 blood volume (replacing approximately 5,000 mL in an adult who weighs 155.56 lb [70 kg]) |
| Replacement fluid for apheresis in thrombotic microangiopathies | Thrombotic thrombocytopenic purpura; hemolytic uremic syndrome |
| Hereditary angioedema | When C1 esterase inhibitor is unavailable9 |
Platelets
Platelet transfusion may be indicated to prevent hemorrhage in patients with thrombocytopenia or platelet function defects. Contraindications to platelet transfusion include thrombotic thrombocytopenic purpura and heparin-induced thrombocytopenia. Transfusion of platelets in these conditions can result in further thrombosis.10,11 One unit of apheresis platelets should increase the platelet count in adults by 30 to 60 × 103 per μL (30 to 60 × 109 per L).3 In neonates, transfusing 5 to 10 mL per kg of platelets should increase the platelet count by 50 to 100 × 103 per μL (50 to 100 × 109 per L).12 One apheresis platelet collection is equivalent to six pooled random donor platelet concentrates.13
Spontaneous bleeding through intact endothelium does not occur unless the platelet count is no greater than 5 × 103 per μL (5 × 109 per L).9 One randomized controlled trial evaluated a threshold for prophylactic platelet transfusion in patients with acute myeloid leukemia.14 Patients were randomized based on platelet transfusion triggers of 10 × 103 per μL (10 × 109 per L) or 20 × 103 per μL (20 × 109 per L). Patients in the lower trigger group received 21.5 percent fewer transfusions than the higher trigger group. Gastrointestinal bleeding was more common in the lower trigger group; however, there was no difference in blood transfusions between groups. Tables 29 and 39–12 give indications for platelet transfusion in adults and neonates, respectively.
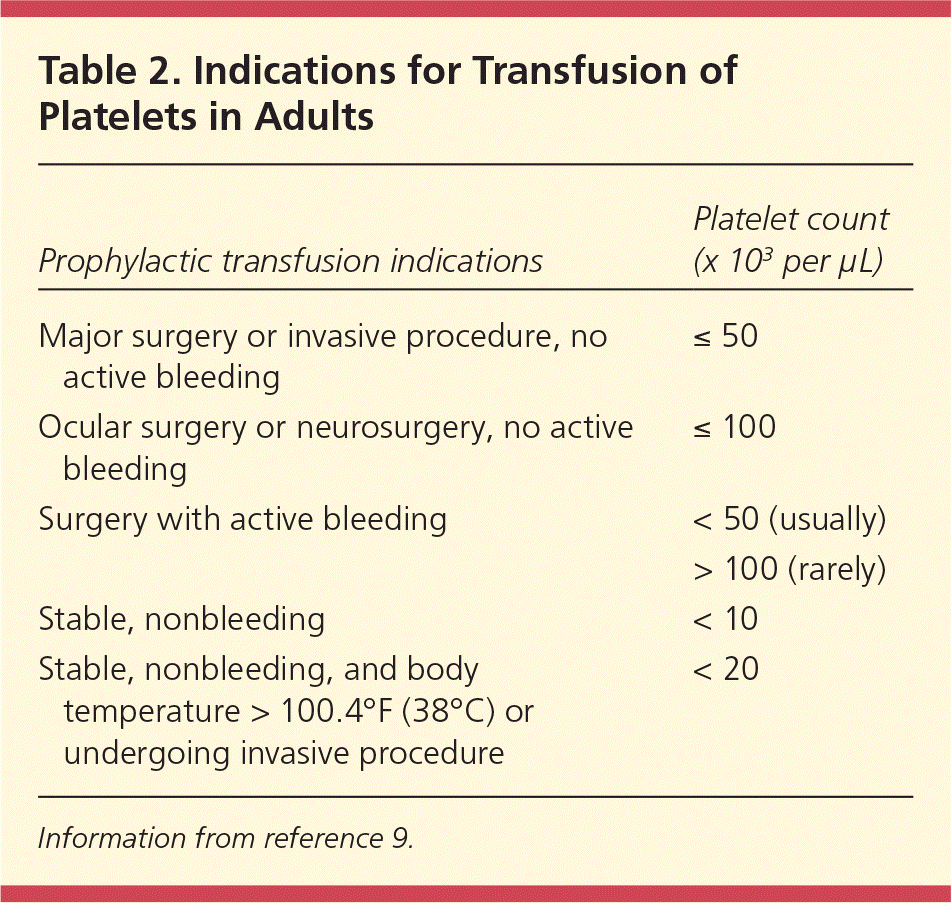
| Prophylactic transfusion indications | Platelet count(× 103 per μL) |
|---|---|
| Major surgery or invasive procedure, no active bleeding | ≤ 50 |
| Ocular surgery or neurosurgery, no active bleeding | ≤ 100 |
| Surgery with active bleeding | < 50 (usually) |
| > 100 (rarely) | |
| Stable, nonbleeding | < 10 |
| Stable, nonbleeding, and body temperature > 100.4°F (38°C) or undergoing invasive procedure | < 20 |

| Platelet count(× 103 per μL) | Indications |
|---|---|
| < 20 | Always transfuse |
| 20 to < 30 | Consider transfusion; transfuse for clinical reasons (e.g., active bleeding, lumbar puncture) |
| 30 to 50 | Transfuse if any of the following indications exist: |
| First week of life with birth weight < 1,000 g (2 lb, 4 oz) | |
| Intraventricular or intraparenchymal cerebral hemorrhage | |
| Coagulation disorder | |
| Sepsis or fluctuating arterial venous pressures | |
| Invasive procedure | |
| Alloimmune neonatal thrombocytopenia* |
Cryoprecipitate
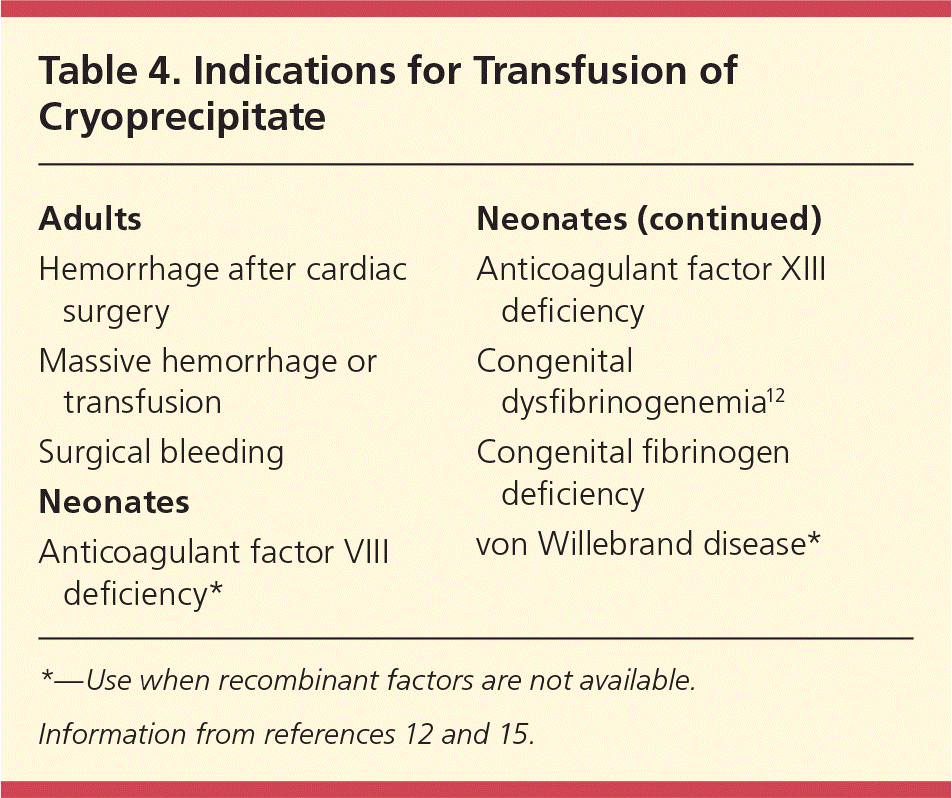
Cryoprecipitate is prepared by thawing fresh frozen plasma and collecting the precipitate. Cryoprecipitate contains high concentrations of factor VIII and fibrinogen. Cryoprecipitate is used in cases of hypofibrinogenemia, which most often occurs in the setting of massive hemorrhage or consumptive coagulopathy. Indications for cryoprecipitate transfusion are listed in Table 4.12,15 Each unit will raise the fibrinogen level by 5 to 10 mg per dL (0.15 to 0.29 μmol per L), with the goal of maintaining a fibrinogen level of at least 100 mg per dL (2.94 μmol per L).15 The usual dose in adults is 10 units of pooled cryoprecipitate.3,15 Recommendations for dosing regimens in neonates vary, ranging from 2 mL of cryoprecipitate per kg to 1 unit of cryoprecipitate (15 to 20 mL) per 7 kg.12
Transfusion Complications
Transfusion-related complications can be categorized as acute or delayed, which can be divided further into the categories of noninfectious (Table 516 ) and infectious (Table 616,17 ). Acute complications occur within minutes to 24 hours of the transfusion, whereas delayed complications may develop days, months, or even years later. The AABB (formerly known as the American Association of Blood Banks) uses the term “noninfectious serious hazards of transfusion” to classify noninfectious complications.16 Transfusion-related infections are less common because of advances in the blood screening process; the risk of contracting an infection from transfusion has decreased 10,000-fold since the 1980s.17 Noninfectious serious hazards of transfusion are up to 1,000 times more likely than an infectious complication.16 However, there has been no progress in preventing noninfectious serious hazards of transfusion, despite improvements in blood screening tests and other related medical advances. Therefore, patients are far more likely to experience a noninfectious serious hazard of transfusion than an infectious complication.17

| Acute |
| Acute hemolytic reaction |
| Allergic reaction |
| Anaphylactic reaction |
| Coagulation problems in massive transfusion |
| Febrile nonhemolytic reaction |
| Metabolic derangements |
| Mistransfusion (transfusion of the incorrect product to the incorrect recipient) |
| Septic or bacterial contamination |
| Transfusion-associated circulatory overload |
| Transfusion-related acute lung injury |
| Urticarial reaction |
| Delayed |
| Delayed hemolytic reaction |
| Iron overload |
| Microchimerism |
| Overtransfusion or undertransfusion |
| Post-transfusion purpura |
| Transfusion-associated graft-versus-host disease |
| Transfusion-related immunomodulation |
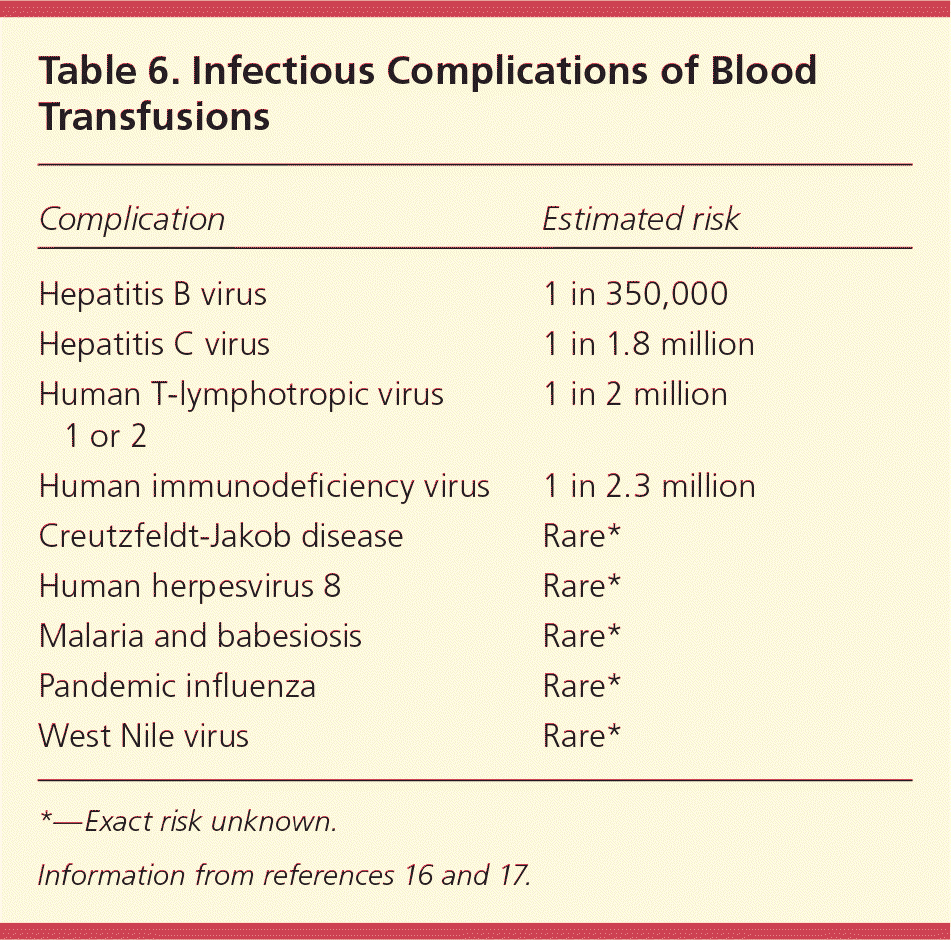
| Complication | Estimated risk |
|---|---|
| Hepatitis B virus | 1 in 350,000 |
| Hepatitis C virus | 1 in 1.8 million |
| Human T-lymphotropic virus 1 or 2 | 1 in 2 million |
| Human immunodeficiency virus | 1 in 2.3 million |
| Creutzfeldt-Jakob disease | Rare* |
| Human herpesvirus 8 | Rare* |
| Malaria and babesiosis | Rare* |
| Pandemic influenza | Rare* |
| West Nile virus | Rare* |
Acute Transfusion Reactions
ACUTE HEMOLYTIC REACTIONS
Hemolytic transfusion reactions are caused by immune destruction of transfused RBCs, which are attacked by the recipient's antibodies. The antibodies to the antigens of the ABO blood group or alloantibodies to other RBC antigens are produced after immunization through a previous transfusion or pregnancy. There are two categories of hemolytic transfusion reactions: acute and delayed. Nonimmune causes of acute reactions include bacterial overgrowth, improper storing, infusion with incompatible medications, and infusion of blood through lines containing hypotonic solutions or small-bore intravenous tubes.16,18,19
In acute hemolytic transfusion reactions, there is a destruction of the donor's RBCs within 24 hours of transfusion. Hemolysis may be intravascular or extravascular. The most common type is extravascular hemolysis, which occurs when donor RBCs coated with immunoglobulin G (IgG) or complement are attacked in the liver or spleen.17 Intravascular hemolysis is a severe form of hemolysis caused by ABO antibodies. Symptoms of acute hemolytic transfusion reactions include fever, chills, rigors, nausea, vomiting, dyspnea, hypotension, diffuse bleeding, hemoglobinuria, oliguria, anuria, pain at the infusion site; and chest, back, and abdominal pain.19 Associated complications are clinically significant anemia, acute or exacerbated renal failure, disseminated intravascular coagulation, need for dialysis, and death secondary to complications.18
ALLERGIC REACTIONS
Allergic reactions range from mild (urticarial) to life threatening (anaphylactic). Urticarial allergic reactions are defined by hives or pruritus.20 Patients experiencing allergic transfusion reactions have been sensitized to the antigens in the donor unit. These antigens are soluble, and the associated reaction is dose-dependent. Allergic transfusion reactions occur in 1 to 3 percent of transfusions.16
Patients with anaphylactic transfusion reactions, like those with urticarial reactions, may present with hives, but they are distinct in that they also develop hypotension, bronchospasm, stridor, and gastrointestinal symptoms.16 Anaphylaxis occurs in response to a recipient's presensitization to a variety of proteins in donor plasma. For example, anaphylaxis occurs because of donor IgA being infused into a recipient who is IgA deficient and has preexisting circulating anti-IgA.17 In addition, anti–human leukocyte antigen (HLA) antibodies and anticomplement antibodies have been linked to anaphylactic reactions, which are estimated to occur in one in 20,000 to 50,000 transfusions.21
Prevention of anaphylactic transfusion reactions includes avoiding plasma transfusions with IgA in patients known to be IgA deficient. Cellular products (e.g., RBCs, platelets) may be washed to remove plasma in patients with an IgA deficiency.16 The best precaution is observation of the patient during the initial 15 minutes of transfusion.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Transfusion-related acute lung injury (TRALI) is noncardiogenic pulmonary edema causing acute hypoxemia that occurs within six hours of a transfusion and has a clear temporal relationship to the transfusion.22 Patients with TRALI do not have any other risk factors for acute lung injury. Antineutrophil cytoplasmic antibodies or anti-HLA antibodies activate the recipient's immune system, resulting in massive pulmonary edema.17,23 Activated neutrophils in the lungs may also secrete proteolytic enzymes, leading to more tissue damage.24 Optimal methods for detecting these antibodies in donated products have yet to be determined.16
Donor products that contain large amounts of plasma from multiparous women are associated with TRALI. Mortality in the United Kingdom decreased significantly after donor plasma from men was used exclusively.17 In 2006, TRALI was the leading cause of transfusion-related mortality, contributing to 50.7 percent of transfusion-related deaths.16 The TRALI working group of the AABB recommends using male-predominant plasma for transfusions.17 Because this policy excludes a large number of female donors, maintaining an adequate supply of plasma and platelets is a concern.
FEBRILE NONHEMOLYTIC TRANSFUSION REACTIONS
An FNHTR is defined as a rise in body temperature of at least 1.8°F (1°C) above 98.6°F (37°C) within 24 hours after a transfusion; it may involve rigors, chills, and discomfort.10 The fever occurs more often in patients who have been transfused repeatedly and in patients who have been pregnant.25 Leukoreduction, which is the removal or filtration of white blood cells from donor blood, has decreased FNHTR rates.26 FNHTRs are caused by platelet transfusions more often than RBC transfusions and have an incidence that ranges from less than 1 percent to more than 35 percent.16
Two mechanisms have been proposed to explain FNHTRs: a release of antibody-mediated endogenous pyrogen, and a release of cytokines. Common cytokines that may be associated with FNHTRs include interleukin-1, interleukin-6, interleukin-8, and tumor necrosis factor.25 FNHTR is a diagnosis of exclusion that can be made only after ruling out other causes of fever (e.g., hemolysis, sepsis).
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Transfusion-associated circulatory overload is the result of a rapid transfusion of a blood volume that is more than what the recipient's circulatory system can handle. It is not associated with an antibody-mediated reaction. Those at highest risk are recipients with underlying cardiopulmonary compromise, renal failure, or chronic anemia, and infants or older patients.17 Signs and symptoms include tachycardia, cough, dyspnea, hypertension, elevated central venous pressure, elevated pulmonary wedge pressure, and widened pulse pressure. Cardiomegaly and pulmonary edema are often seen on chest radiography.27
The diagnosis is made clinically, but may be assisted by measuring brain natriuretic peptide levels, which are elevated in response to an increase in filling pressure.28 A study comparing patients who have transfusion-associated circulatory overload with patients who have TRALI found significantly greater levels of brain natriuretic peptide in those with transfusion-associated circulatory overload.28 Transfusion of lower volumes or at a slower rate may help prevent it.16 The treatment is diuresis to decrease volume overload.
Delayed Transfusion Reactions
TRANSFUSION-ASSOCIATED GRAFT-VERSUS-HOST DISEASE
Transfusion-associated graft-versus-host disease is a consequence of a donor's lymphocytes proliferating and causing an immune attack against the recipient's tissues and organs. It is fatal in more than 90 percent of cases.16 Patients vulnerable to this condition are those who are immunocompromised or immunocompetent and who are receiving transfusion with shared HLA haplotypes (i.e., donor is a relative).17 Symptoms include rash, fever, diarrhea, liver dysfunction, and pancytopenia occurring one to six weeks after transfusion.16
Risk factors include a history of fludarabine (Oforta) treatment, Hodgkin disease, stem cell transplant, intensive chemotherapy, intrauterine transfusion, or erythroblastosis fetalis. Other probable risk factors include a history of solid tumors treated with cytotoxic drugs, transfusion in premature infants, and recipient-donor pairs from homogenous populations.29 Gamma irradiation of blood products keeps the donor lymphocytes from proliferating and can prevent transfusion-associated graft-versus-host disease.16
