
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2011;83(12):1443-1451
A more recent article on HIV-associated complications is available.
Related letter: Antiretroviral Therapy Regimens in Treatment-Naive Patients
Patient information: See related handout on side effects of antiretroviral therapy, written by the author of this article.
Author disclosure: No relevant financial affiliations to disclose.
Family physicians are treating patients infected with human immunodeficiency virus in their practices more often. Long-term complications of this disease are multifactorial and can be related to the virus itself or to adverse effects of antiretroviral therapy. Each drug class has side effects: nucleoside/nucleotide reverse transcriptase inhibitors are associated with lactic acidosis, lipodystrophy, and hyperlipidemia; non-nucleoside reverse transcriptase inhibitors are associated with neuropsychiatric symptoms, rash, liver toxicity, and lipid abnormalities; and protease inhibitors are associated with gastrointestinal intolerance and glucose and lipid abnormalities. The entry inhibitor maraviroc and the integrase inhibitor raltegravir have been approved for treatment-naive and treatment-experienced patients. Maraviroc is associated with bronchitis, nasopharyngitis, and esophageal candidiasis. Adverse effects of raltegravir are comparable to those experienced with placebo, with the exception of increased risk of myopathy and rhabdomyolysis. Information about drug interactions for both of these medications is limited. Non-nucleoside reverse transcriptase inhibitors and protease inhibitors are primarily metabolized through the cytochrome P450 system, and as a result have numerous drug-drug interactions. Monitoring for adverse effects of antiretroviral therapy includes a complete blood count and comprehensive metabolic profile every three to six months. A lipid profile and urinalysis for proteinuria should be performed annually. Dual energy x-ray absorptiometry should be considered in patients older than 50 years. Long-term morbidity related to antiretroviral therapy includes liver, renal, glucose, and lipid abnormalities, and cardiovascular and bone disease. With some exceptions for lipid management, these morbidities can be managed as in the general population.
Survival rates in patients with human immunodeficiency virus (HIV) infection are improved with early treatment; therefore, current recommendations are to begin antiretroviral therapy (ART) in patients with a CD4 cell count of 500 per mm3 (0.50 × 109 per L) or less1–4 (Table 11,4 ). Because of this new threshold, family physicians can expect to see patients with HIV infection who have been taking ART for longer lengths of time. Pill counts and frequency of dosing have decreased to one to three pills per day for usual starting regimens. Despite this, ART regimens continue to have significant adverse effects that require monitoring for drug interactions and long-term morbidity related to cardiovascular, bone, and kidney disease.
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| Antiretroviral therapy is recommended in patients with CD4 cell counts of 500 per mm3 (0.50 × 109 per L) or less. | C | 1–4 |
| Patients taking antiretroviral therapy should be screened for cardiac risk factors (e.g., dyslipidemia, diabetes mellitus) annually or after a change in therapy, or more frequently if clinically indicated. | C | 1, 5, 8, 16 |
| Dual energy x-ray absorptiometry should be considered in men and women 50 years and older who are taking tenofovir (Viread) or didanosine (Videx). Vitamin D levels should be monitored if clinically indicated. | C | 5, 8 |
| Annual screening for renal disease should include glomerular filtration rate and screening for proteinuria. Black patients; those with CD4 cell counts less than 200 per mm3 (0.20 × 109 per L); those with viral loads greater than 4,000 copies per mL; and those with diabetes, hypertension, or co-occurring hepatitis C should be screened every six months. | C | 5, 39 |

| Recommendations of the Panel on Antiretroviral Guidelines for Adults and Adolescents1 | ||
| Initiate therapy for patients with: | ||
| CD4 cell count < 350 per mm3 (0.35 × 109 per L)* | ||
| Hepatitis B virus coinfection when treatment is indicated | ||
| History of AIDS-defining illness | ||
| HIV-associated nephropathy | ||
| Pregnancy | ||
| Recommendations of the International AIDS Society USA Panel4 | ||
| Initiate therapy for patients with: | ||
| Symptomatic HIV disease | ||
| Asymptomatic HIV disease and CD4 cell count = 500 per mm3(0.50 × 109 per L) | ||
| Asymptomatic HIV disease with: | ||
| Active hepatitis B or C coinfection | ||
| Age > 60 years | ||
| Cardiovascular disease or high risk of cardiovascular disease | ||
| High risk of HIV transmission | ||
| HIV-associated nephropathy | ||
| Pregnancy | ||
| Rapid decline in CD4 cell count (> 100 per mm3 [0.10 × 109 per L] per year) | ||
| Viral load > 100,000 copies per mL | ||
General Principles of ART
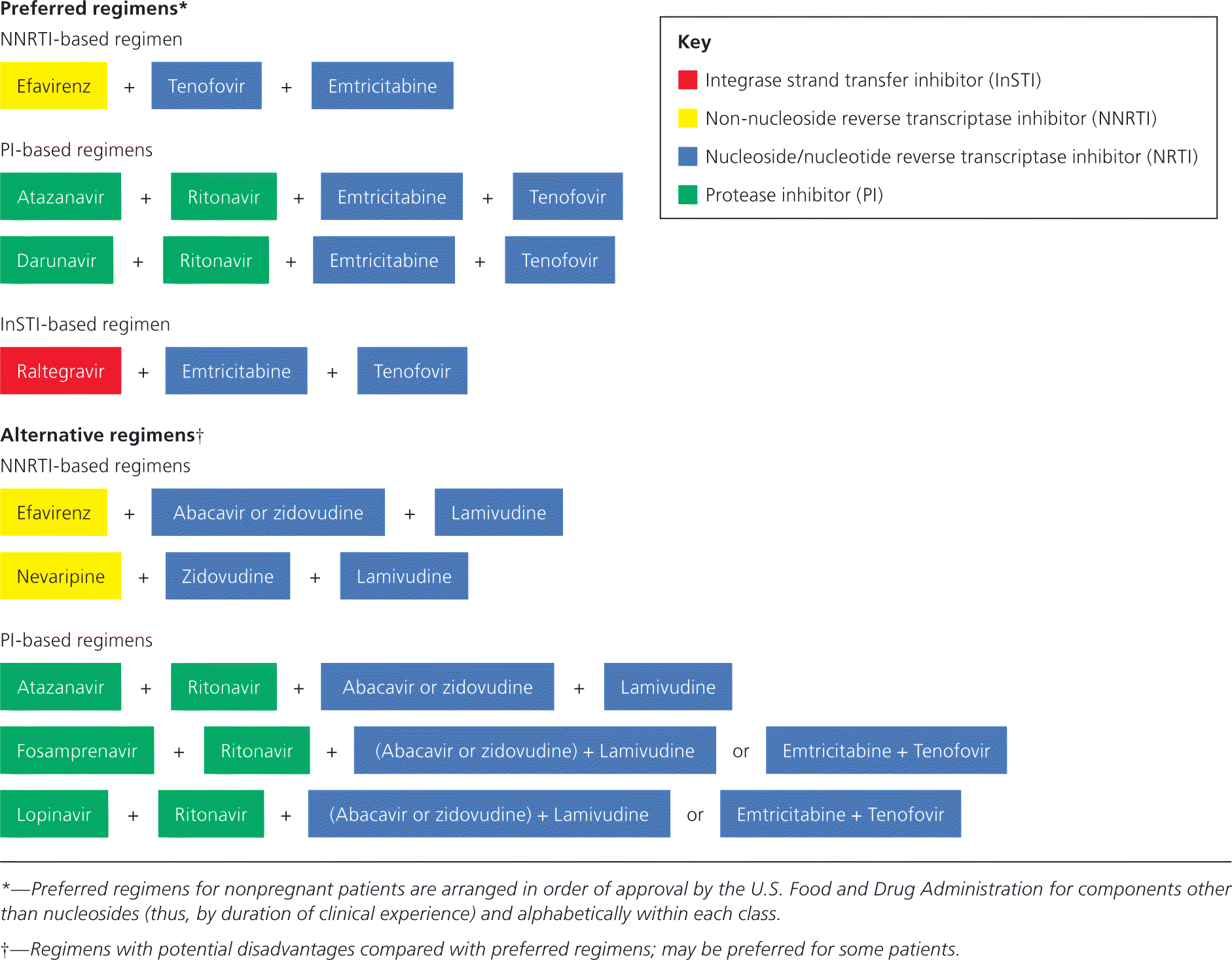
The basic configuration of antiretroviral regimens is unchanged. The most common initial regimens are a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI) with two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs). Figure 1 shows the current preferred initial regimens.1 Common toxicities of ART can make adherence to therapy difficult. However, adherence is important to prevent the development of drug resistance. Unlike therapy for other diseases, a strategy of decreasing the dose or switching to a different drug to minimize toxicity and maximize adherence may not be possible with ART; the benefit of suppressing HIV may override other considerations. Identification and awareness of ART toxicity are necessary to facilitate patient adherence and determine when a change in therapy may be needed.
Routine monitoring in patients receiving ART includes a complete blood count and a comprehensive metabolic panel every three to six months. A lipid profile and urinalysis for proteinuria should be performed annually. When ART is changed, a complete blood count, metabolic panel, and lipid profile should be performed two to eight weeks afterward. Abnormal results should prompt more frequent testing based on clinical assessment.1,4,5
There are several drug-drug interactions between ART and other medications. NNRTIs, PIs, and the entry inhibitor maraviroc (Selzentry) have the most interactions because they are metabolized through the cytochrome P450 (CYP450) system. A comprehensive list of interactions is available at http://aidsinfo.nih.gov/contentfiles/AA_Tables.pdf, Tables 13 and 15a through 15e. Common interactions are listed in Table 2.1,5,6 Physicians should check for drug interactions when a new medication is initiated and check current medications when a change is made in ART.
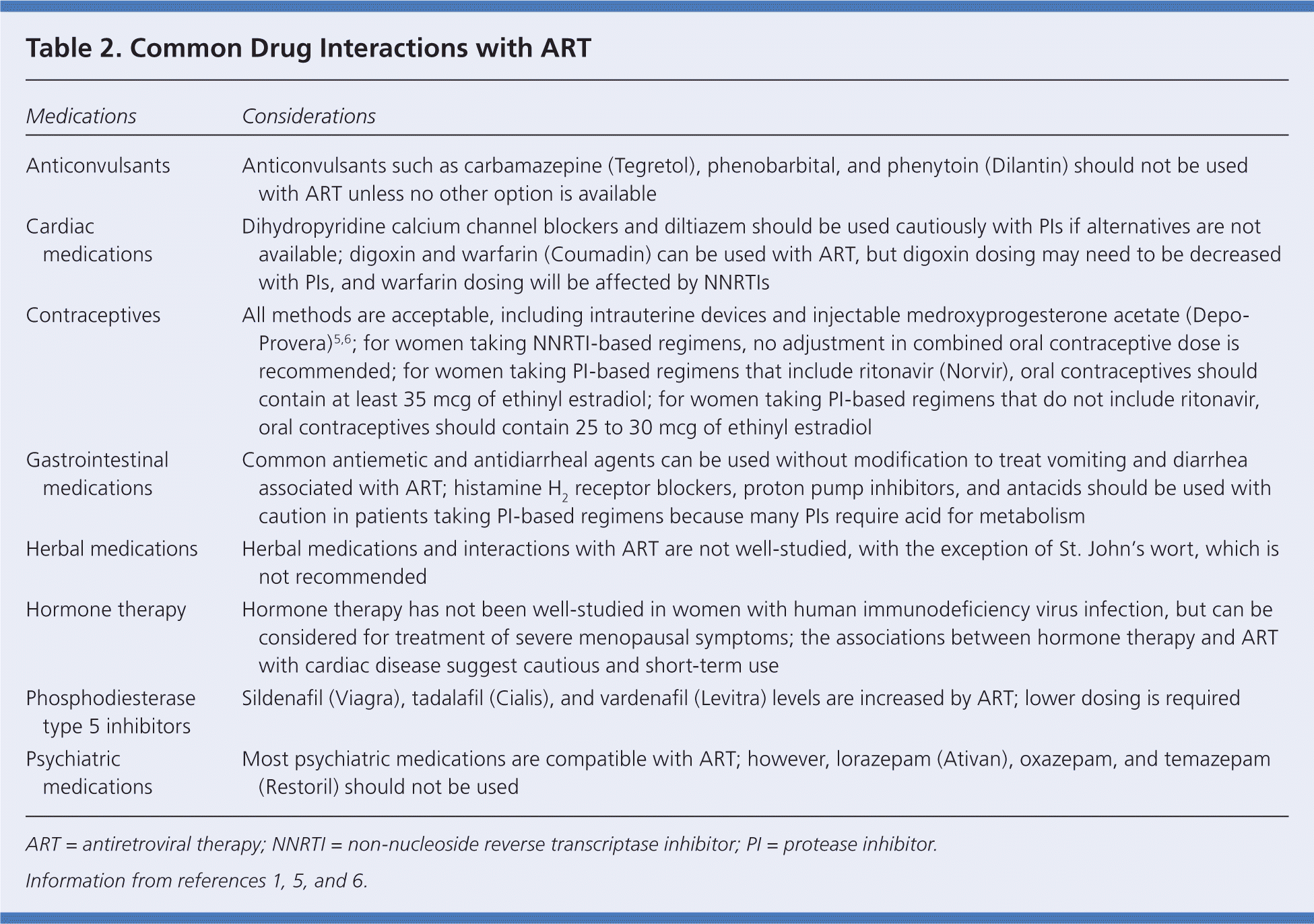
| Medications | Considerations |
|---|---|
| Anticonvulsants | Anticonvulsants such as carbamazepine (Tegretol), phenobarbital, and phenytoin (Dilantin) should not be used with ART unless no other option is available |
| Cardiac medications | Dihydropyridine calcium channel blockers and diltiazem should be used cautiously with PIs if alternatives are not available; digoxin and warfarin (Coumadin) can be used with ART, but digoxin dosing may need to be decreased with PIs, and warfarin dosing will be affected by NNRTIs |
| Contraceptives | All methods are acceptable, including intrauterine devices and injectable medroxyprogesterone acetate (Depo-Provera)5,6; for women taking NNRTI-based regimens, no adjustment in combined oral contraceptive dose is recommended; for women taking PI-based regimens that include ritonavir (Norvir), oral contraceptives should contain at least 35 mcg of ethinyl estradiol; for women taking PI-based regimens that do not include ritonavir, oral contraceptives should contain 25 to 30 mcg of ethinyl estradiol |
| Gastrointestinal medications | Common antiemetic and antidiarrheal agents can be used without modification to treat vomiting and diarrhea receptor blockers, proton pump inhibitors, and antacids should be used with associated with ART; histamine H2 caution in patients taking PI-based regimens because many PIs require acid for metabolism |
| Herbal medications | Herbal medications and interactions with ART are not well-studied, with the exception of St. John's wort, which is not recommended |
| Hormone therapy | Hormone therapy has not been well-studied in women with human immunodeficiency virus infection, but can be considered for treatment of severe menopausal symptoms; the associations between hormone therapy and ART with cardiac disease suggest cautious and short-term use |
| Phosphodiesterase type 5 inhibitors | Sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra) levels are increased by ART; lower dosing is required |
| Psychiatric medications | Most psychiatric medications are compatible with ART; however, lorazepam (Ativan), oxazepam, and temazepam (Restoril) should not be used |
Drug Classes
NUCLEOSIDE/NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS
NRTIs, the backbone of ART, are associated with lactic acidosis and lipodystrophy (Table 3).1,7 Symptoms of lactic acidosis include fatigue, nausea and vomiting, abdominal pain, diarrhea, and an increase in liver function markers because of hepatic steatosis. A serum venous lactate level can be used to screen for this complication, but should be confirmed by arterial blood gas testing. If venous or arterial lactate levels are more than 45.05 mg per dL (5.00 mmol per L), the NRTI should be cautiously changed to a nonthymidine NRTI.5,8 Lipodystrophy includes lipoatrophy (i.e., loss of subcutaneous fat in the face and extremities) and lipohypertrophy (i.e., dorsocervical fat pad and central abdominal fat accumulation). Polylactic acid and calcium hydroxylapatite injections can provide short-term benefit for facial lipoatrophy5,8; and liposuction and administration of recombinant growth hormone may provide short-term benefit for lipohypertrophy.5,8 Diet changes, including a decrease in polyunsaturated fat intake and an increase in fiber intake, as well as the use of metformin (Glucophage), may decrease lipohypertrophy.8
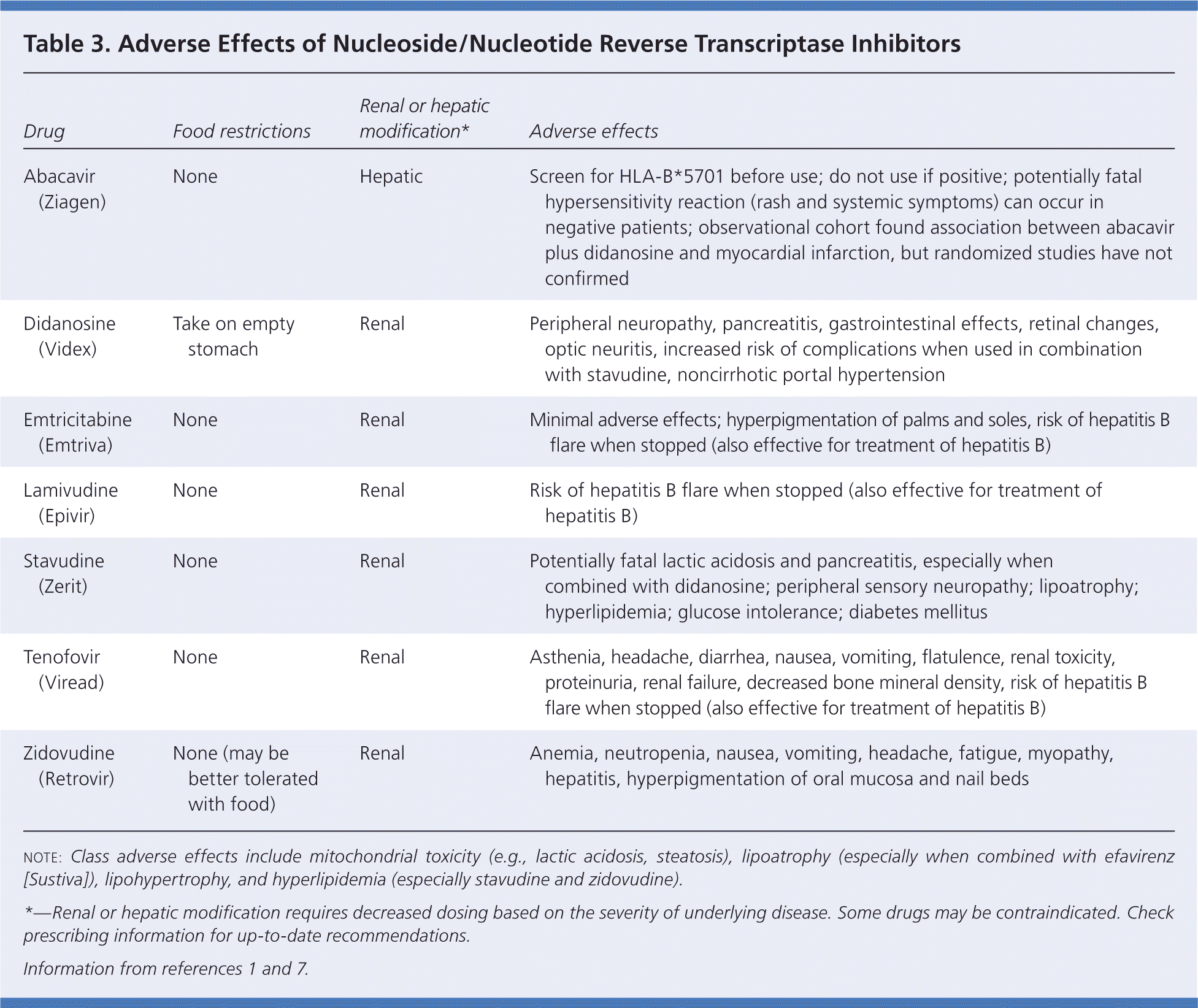
| Drug | Food restrictions | Renal or hepatic modification* | Adverse effects |
|---|---|---|---|
| Abacavir (Ziagen) | None | Hepatic | Screen for HLA-B*5701 before use; do not use if positive; potentially fatal hypersensitivity reaction (rash and systemic symptoms) can occur in negative patients; observational cohort found association between abacavir plus didanosine and myocardial infarction, but randomized studies have not confirmed |
| Didanosine (Videx) | Take on empty stomach | Renal | Peripheral neuropathy, pancreatitis, gastrointestinal effects, retinal changes, optic neuritis, increased risk of complications when used in combination with stavudine, noncirrhotic portal hypertension |
| Emtricitabine (Emtriva) | None | Renal | Minimal adverse effects; hyperpigmentation of palms and soles, risk of hepatitis B flare when stopped (also effective for treatment of hepatitis B) |
| Lamivudine (Epivir) | None | Renal | Risk of hepatitis B flare when stopped (also effective for treatment of hepatitis B) |
| Stavudine (Zerit) | None | Renal | Potentially fatal lactic acidosis and pancreatitis, especially when combined with didanosine; peripheral sensory neuropathy; lipoatrophy; hyperlipidemia; glucose intolerance; diabetes mellitus |
| Tenofovir (Viread) | None | Renal | Asthenia, headache, diarrhea, nausea, vomiting, flatulence, renal toxicity, proteinuria, renal failure, decreased bone mineral density, risk of hepatitis B flare when stopped (also effective for treatment of hepatitis B) |
| Zidovudine (Retrovir) | None (may be better tolerated with food) | Renal | Anemia, neutropenia, nausea, vomiting, headache, fatigue, myopathy, hepatitis, hyperpigmentation of oral mucosa and nail beds |
The most commonly used NRTIs are zidovudine (Retrovir), lamivudine (Epivir), emtricitabine (Emtriva), and tenofovir (Viread). Adverse effects associated with zidovudine include bone marrow suppression, nausea and vomiting, fatigue, and headache. Lamivudine and emtricitabine are well tolerated. However, in patients with co-occurring HIV and hepatitis B infections, discontinuing these medications can cause a flare of hepatitis B.
Tenofovir is a common NRTI in initial regimens and is taken with lamivudine or emtricitabine. Tenofovir combined with emtricitabine (Truvada) allows once-daily dosing. Tenofovir has been associated with renal abnormalities such as glycosuria, hypophosphatemia, and increased creatinine levels, and the potential for acute tubular necrosis. However, there is uncertainty about the role of tenofovir in renal dysfunction. The risk of acute renal failure in patients taking tenofovir is estimated to be 1 percent; this risk may be increased in those with advanced HIV disease, greater treatment experience, or preexisting renal disease.9 Cumulative tenofovir exposure may increase the risk of chronic kidney disease, but the increase is similar to the risk associated with cumulative atazanavir (Reyataz) and lopinavir/ritonavir (Kaletra) use.10
NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
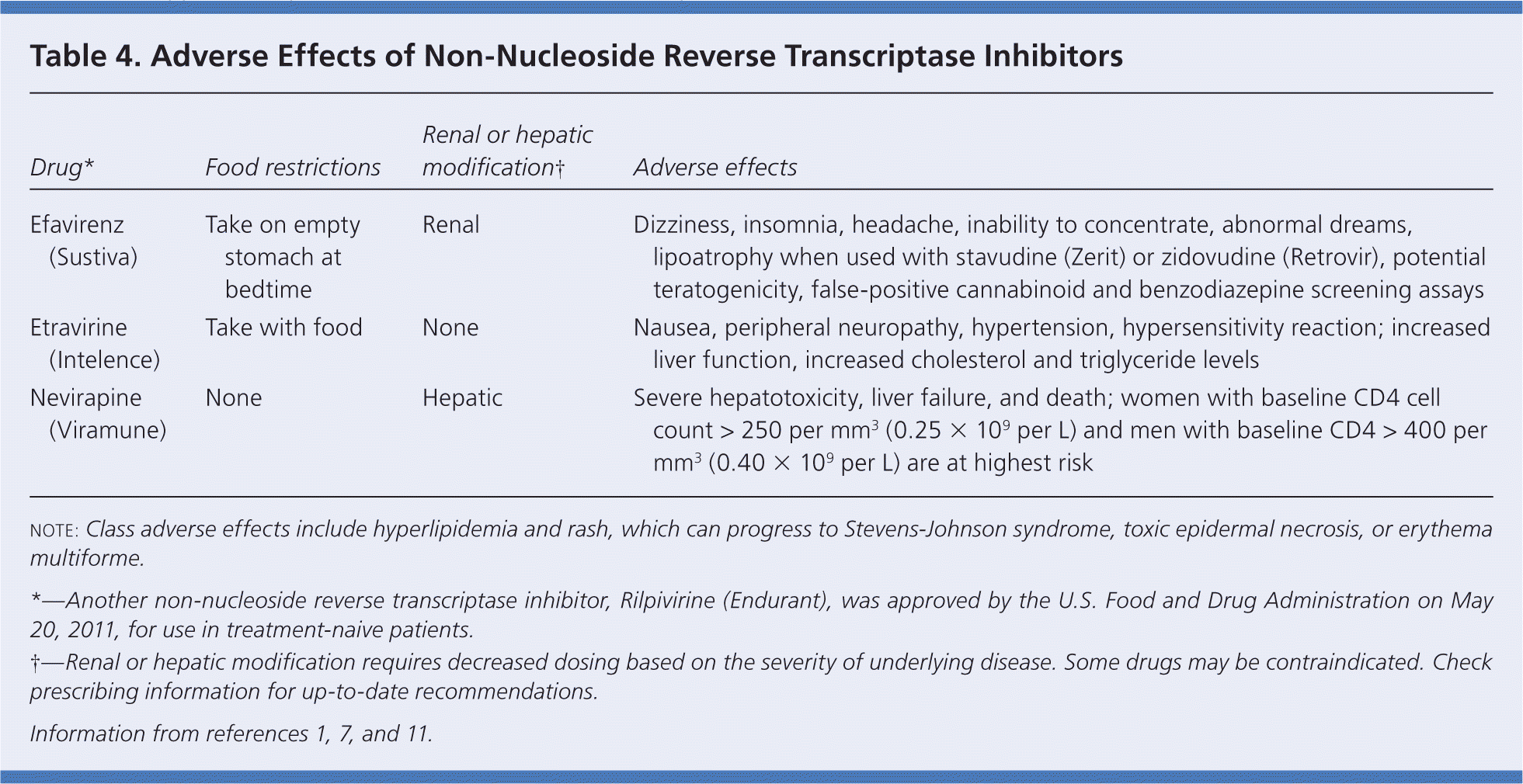
| Drug* | Food restrictions | Renal or hepatic modification | Adverse effects |
|---|---|---|---|
| Efavirenz (Sustiva) | Take on empty stomach at bedtime | Renal | Dizziness, insomnia, headache, inability to concentrate, abnormal dreams, lipoatrophy when used with stavudine (Zerit) or zidovudine (Retrovir), potential teratogenicity, false-positive cannabinoid and benzodiazepine screening assays |
| Etravirine (Intelence) | Take with food | None | Nausea, peripheral neuropathy, hypertension, hypersensitivity reaction; increased liver function, increased cholesterol and triglyceride levels |
| Nevirapine (Viramune) | None | Hepatic | Severe hepatotoxicity, liver failure, and death; women with baseline CD4 cell count > 250 per mm3 (0.25 × 109 per L) and men with baseline CD4 > 400 per mm3 (0.40 × 109 per L) are at highest risk |
Nevirapine (Viramune) and efavirenz (Sustiva) are first-generation NNRTIs in common use. However, nevirapine is limited by its association with severe hepatotoxicity and liver failure in women with a baseline CD4 cell count of more than 250 per mm3 (0.25 × 109 per L) and in men with a baseline count of more than 400 per mm3 (0.40 × 109 per L). Efavirenz is the most commonly used NNRTI, and is combined in a once-daily pill with emtricitabine and tenofovir (Atripla). Efavirenz can cause adverse effects of the central nervous system (e.g., sleep, cognitive, and mood disorders) in 50 percent of patients during the first month of treatment; this prevalence decreases to 23 percent at three months, and is similar to baseline by six months.1,13,14 Efavirenz also can cause hyperlipidemia and liver toxicity, and is not recommended for use during pregnancy.
Etravirine (Intelence) is the first second-generation NNRTI.15 It has been studied primarily in treatment-experienced patients. Resistance, including intra-class resistance, has developed to first-generation NNRTIs with a single mutation (K103N). Multiple mutations are required before resistance to etravirine develops, and it can be used in patients with resistance to other NNRTIs caused by K103N mutation. The most common adverse effects are rash and nausea. The liver, lipid, and neuropsychiatric effects common with first-generation NNRTIs have not been reported with etravirine. Development of a rash (19.5 percent of patients) is an indication for stopping the medication and not rechallenging; severe and possibly fatal Stevens-Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme occur in 1.3 percent of patients taking etravirine.15 Women may be at higher risk of rash than men (30 versus 18 percent).15 Etravirine is metabolized by the CYP450 system, so drug interactions will be significant as they are identified.
PROTEASE INHIBITORS
Several PIs are currently in use (Table 5).1,7,16 Boosting refers to the use of a small dose of ritonavir (Norvir) in combination with another PI. Most ART regimens are boosted, although some patients cannot tolerate the adverse gastrointestinal effects of ritonavir. PIs are metabolized by the CYP450 system and are associated with the most drug-drug interactions. Common adverse effects of PIs include gastrointestinal effects, lipohypertrophy, glucose intolerance or diabetes mellitus, and lipid disorders.
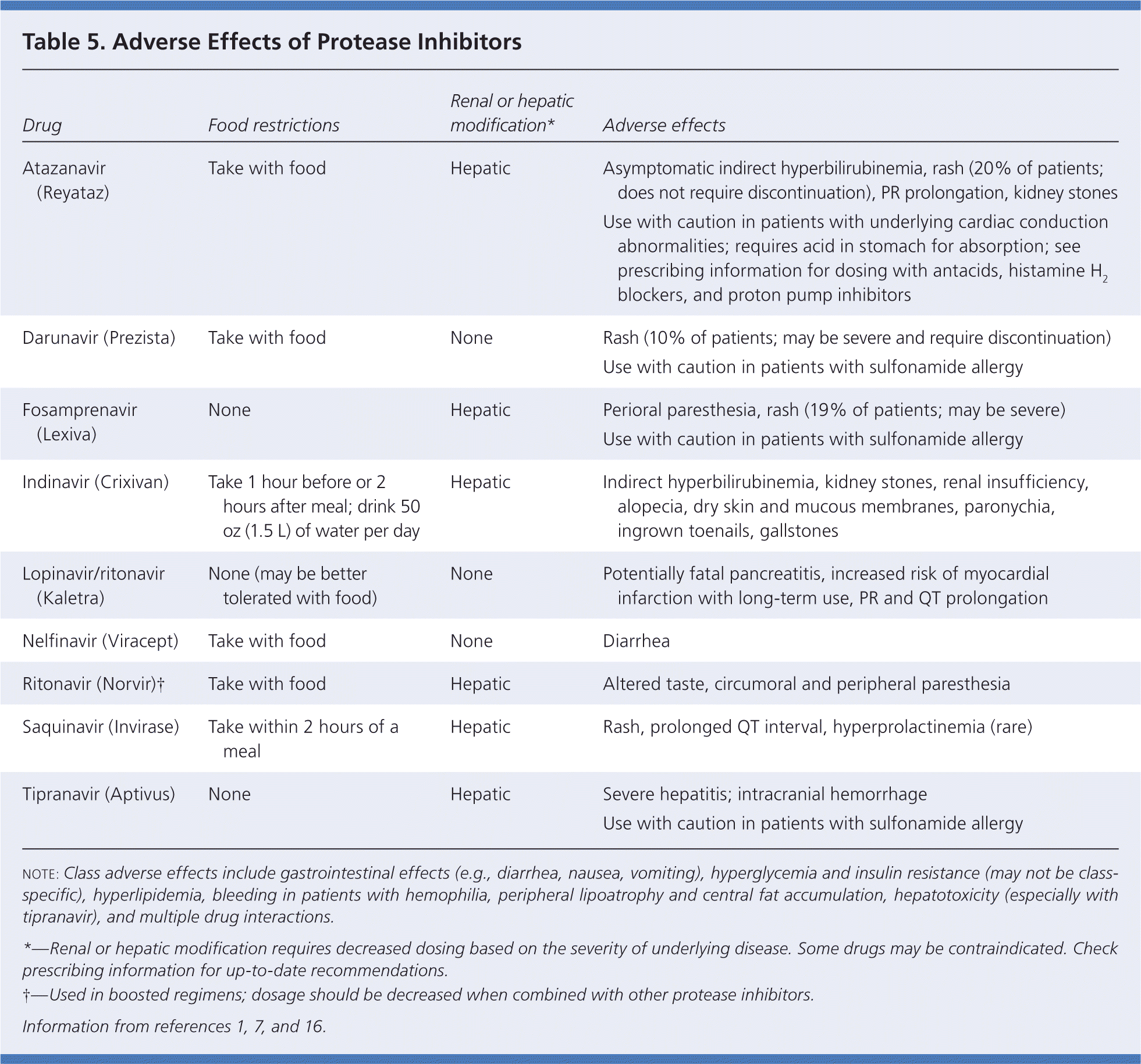
| Drug | Food restrictions | Renal or hepatic modification* | Adverse effects |
|---|---|---|---|
| Atazanavir (Reyataz) | Take with food | Hepatic | Asymptomatic indirect hyperbilirubinemia, rash (20% of patients; does not require discontinuation), PR prolongation, kidney stones |
| Use with caution in patients with underlying cardiac conduction abnormalities; requires acid in stomach for absorption; see prescribing information for dosing with antacids, histamine H2 blockers, and proton pump inhibitors | |||
| Darunavir (Prezista) | Take with food | None | Rash (10% of patients; may be severe and require discontinuation) |
| Use with caution in patients with sulfonamide allergy | |||
| Fosamprenavir (Lexiva) | None | Hepatic | Perioral paresthesia, rash (19% of patients; may be severe) Use with caution in patients with sulfonamide allergy |
| Indinavir (Crixivan) | Take 1 hour before or 2 hours after meal; drink 50 oz (1.5 L) of water per day | Hepatic | Indirect hyperbilirubinemia, kidney stones, renal insufficiency, alopecia, dry skin and mucous membranes, paronychia, ingrown toenails, gallstones |
| Lopinavir/ritonavir (Kaletra) | None (may be better tolerated with food) | None | Potentially fatal pancreatitis, increased risk of myocardial infarction with long-term use, PR and QT prolongation |
| Nelfinavir (Viracept) | Take with food | None | Diarrhea |
| Ritonavir (Norvir) | Take with food | Hepatic | Altered taste, circumoral and peripheral paresthesia |
| Saquinavir (Invirase) | Take within 2 hours of a meal | Hepatic | Rash, prolonged QT interval, hyperprolactinemia (rare) |
| Tipranavir (Aptivus) | None | Hepatic | Severe hepatitis; intracranial hemorrhage Use with caution in patients with sulfonamide allergy |
Approximately 60 percent of patients taking PIs have elevated total cholesterol levels (greater than 240 mg per dL [6.22 mmol per L]), and 75 percent have triglyceride levels greater than 500 mg per dL (5.65 mmol per L).16 Unboosted atazanavir is considered the most lipid-favorable PI; boosted darunavir (Prezista) and boosted atazanavir are the next best choices if baseline hyperlipidemia is significant.1 PIs are associated with glucose intolerance; however this condition is present in 15 to 20 percent of patients with HIV infection, and diabetes develops in up to 6 percent, regardless of PI use.1,5 All medications for diabetes can be used without dosage adjustment in patients with HIV infection.1 Insulin-sensitizing agents, including metformin and thiazolidinediones, are recommended.8 Because PIs are metabolized by the CYP450 system, the choice of statins is important in patients with lipid disorders who are also taking ART (Table 6).5,8,16 Pravastatin (Pravachol) can generally be used without dosage adjustment, but the lowest possible dosage should be used in patients who are also taking darunavir. Rosuvastatin (Crestor) and atorvastatin (Lipitor) are alternative medications. Simvastatin (Zocor) and lovastatin (Mevacor) should not be used with PIs; niacin, fibrates, and omega-3 fatty acids can be used.12
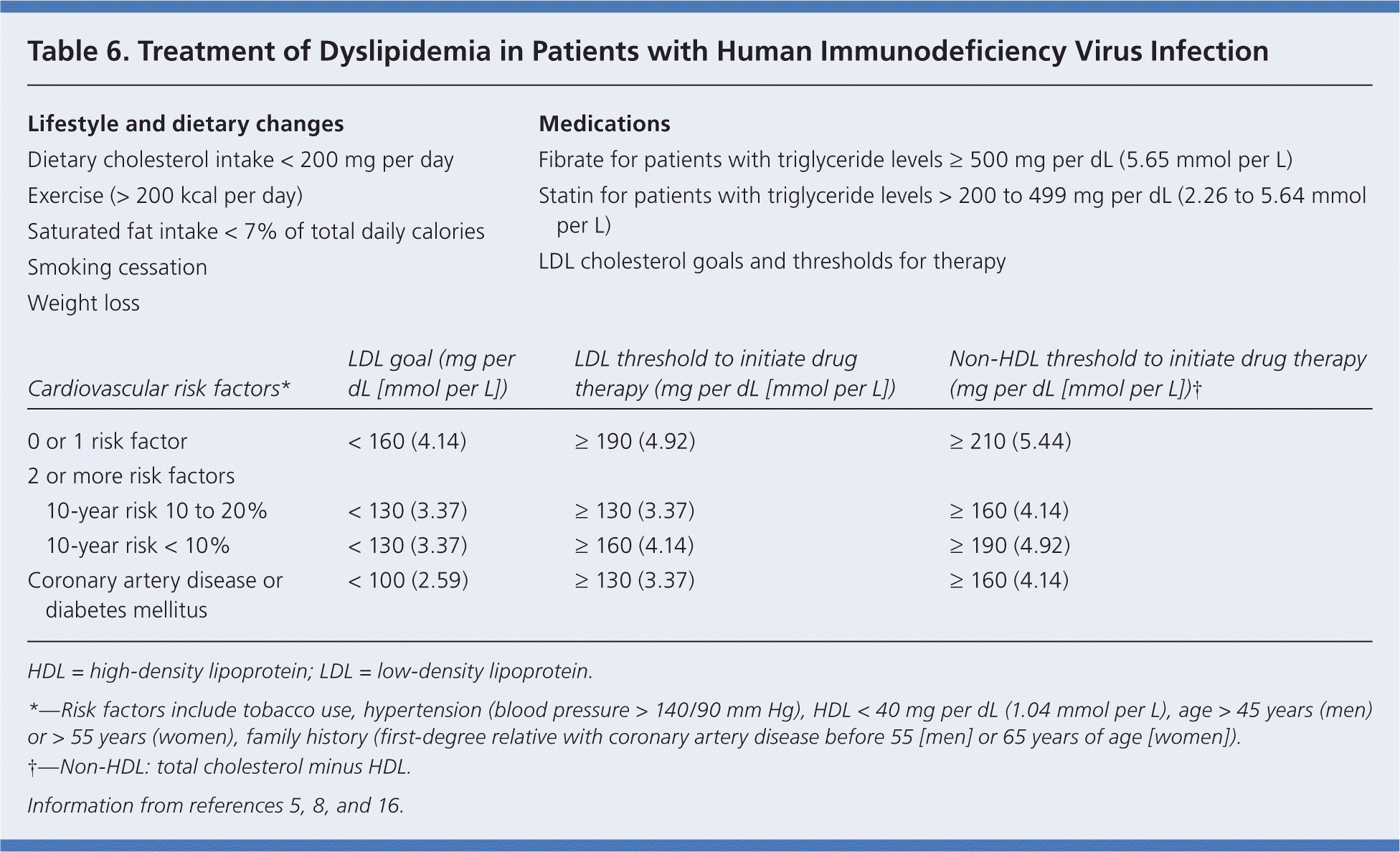
| Lifestyle and dietary changes | Medications | |||
|
| |||
| Cardiovascular risk factors* | LDL goal (mg per dL [mmol per L]) | LDL threshold to initiate drug therapy (mg per dL [mmol per L]) | Non-HDL threshold to initiate drug therapy (mg per dL [mmol per L])† | |
| 0 or 1 risk factor | < 160 (4.14) | ≥ 190 (4.92) | ≥ 210 (5.44) | |
| 2 or more risk factors | ||||
| 10-year risk 10 to 20% | < 130 (3.37) | ≥ 130 (3.37) | ≥160 (4.14) | |
| 10-year risk < 10% | < 130 (3.37) | ≥ 160 (4.14) | ≥ 190 (4.92) | |
| Coronary artery disease or diabetes mellitus | < 100 (2.59) | ≥ 130 (3.37) | ≥ 160 (4.14) | |
ENTRY AND FUSION INHIBITORS
HIV enters the CD4 cell by fusing with the cell membrane and attaching to chemokine receptors. Entry and fusion inhibitors block these receptors, preventing the virus from entering the cells. Enfuvirtide (Fuzeon), a fusion inhibitor, is used primarily in treatment-experienced patients with limited therapeutic options. However, it is a painful subcutaneous injection, and is not commonly used. Common adverse effects include neutropenia and an increased risk of pneumonia (Table 7).1,7,17
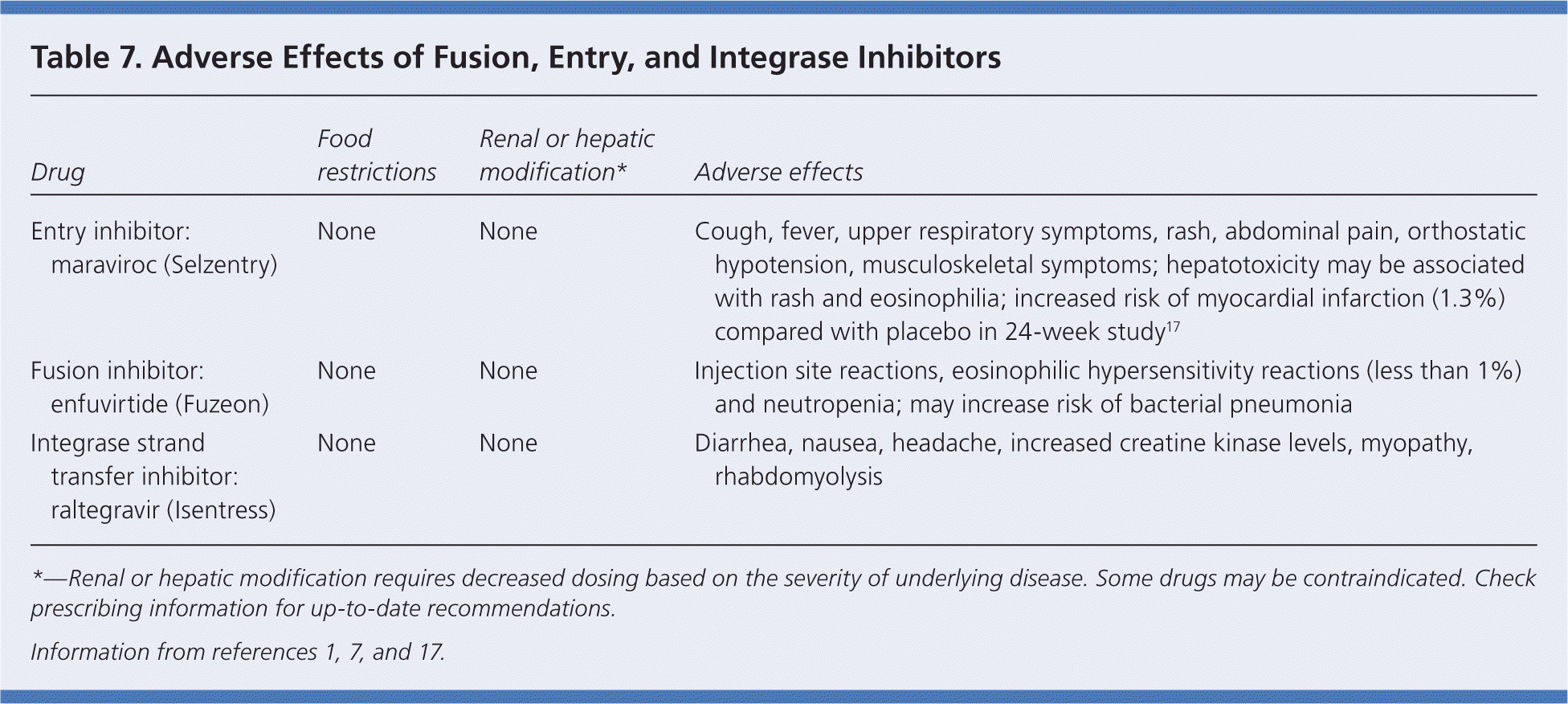
| Drug | Food restrictions | Renal or hepatic modification* | Adverse effects |
|---|---|---|---|
| Entry inhibitor: maraviroc (Selzentry) | None | None | Cough, fever, upper respiratory symptoms, rash, abdominal pain, orthostatic hypotension, musculoskeletal symptoms; hepatotoxicity may be associated with rash and eosinophilia; increased risk of myocardial infarction (1.3%) compared with placebo in 24-week study17 |
| Fusion inhibitor: enfuvirtide (Fuzeon) | None | None | Injection site reactions, eosinophilic hypersensitivity reactions (less than 1%) and neutropenia; may increase risk of bacterial pneumonia |
| Integrase strand transfer inhibitor: raltegravir (Isentress) | None | None | Diarrhea, nausea, headache, increased creatine kinase levels, myopathy, rhabdomyolysis |
C-C chemokine receptors on CD4 cells include CCR5 (R5) and CXCR4 (X4). Maraviroc, an entry inhibitor, is the only available CCR5 antagonist. It is approved for use in both treatment-naive and treatment-experienced patients.18,19 Maraviroc works only on R5 cells; therefore, an R5 tropism test should be performed before initiating therapy with maraviroc.1 Maraviroc is as effective as efavirenz when each drug is combined with lamivudine/zidovudine (Combivir). However, maraviroc is better tolerated; 13.6 percent of patients discontinued therapy with efavirenz because of adverse effects, whereas only 4.2 percent discontinued maraviroc.20 Compared with patients taking efavirenz, those receiving maraviroc have more cases of bronchitis and nasopharyngitis. Patients taking maraviroc also report more fever and esophageal candidiasis, but fewer headache symptoms than those receiving placebo.18 Lipid profiles in patients receiving maraviroc are also better compared with those receiving efavirenz. Studies of interactions with other medications are limited.
INTEGRASE STRAND TRANSFER INHIBITORS
Integrase strand transfer inhibitors prevent viral DNA from integrating into host DNA by inhibiting the integrase enzyme involved in strand transfer.21–24 Raltegravir (Isentress) is the first drug in this class to be approved for use in both treatment-naive and treatment-experienced patients.21–24 It is taken orally twice per day. Resistance to raltegravir develops easily, which may limit its long-term effectiveness. Myopathy and rhabdomyolysis have been reported in patients receiving raltegravir23,24; however, adverse effects are similar to those with placebo. Compared with patients receiving efavirenz, those receiving raltegravir have fewer central nervous system and neuropsychiatric problems.23,24 Studies of interactions with other medications are limited.
Treatment Considerations
Cardiovascular disease accounts for 10 percent of deaths in patients with HIV infection.1 ART that includes indinavir (Crixivan), lopinavir/ritonavir, didanosine (Videx), or abacavir (Ziagen) increases the risk of heart disease.25,26 Multiple studies—with conflicting results—suggest that lower CD4 cell counts, higher viral load, and cumulative exposure to ART are associated with increased cardiovascular risk.1,25,27 Heavy alcohol use (at least 14 drinks per week in men) may increase the risk of cardiovascular disease independently of CD4 cell count, ART, or traditional cardiac risk factors.28 Patients should be screened for traditional cardiac risk factors (e.g., hypertension, diabetes, dyslipidemia, tobacco use, family history) annually or after a change in therapy, or more frequently if clinically indicated, and treated according to guidelines for the general population.1,5,8,16
Vitamin D deficiency, osteopenia, and osteoporosis are more common in patients with HIV infection5,8,29–34; osteopenia occurs in 52 percent of patients and osteoporosis in 15 percent.35 Tenofovir or didanosine use is associated with lower bone mineral density in men and women.29 Vitamin D deficiency is associated with the use of NNRTIs32; screening should be considered in patients receiving these drugs. Osteoporosis screening should begin after 50 years of age in patients receiving tenofovir or didanosine, and is recommended in all patients older than 50 years who have HIV infection if other risk factors are present (other than female sex and menopause).5,8 Bisphosphonates, calcium, and vitamin D can be used for treatment of osteoporosis.36–38
It is estimated that 30 percent of patients with HIV infection have abnormal kidney function.5,39 Many HIV medications require dosage adjustment in patients with glomerular filtration rates less than 50 mL per minute per 1.73 m2; however, NNRTIs and PIs do not.39 Renal insufficiency is related to ART (tenofovir, indinavir, atazanavir, lopinavir/ritonavir) but also to HIV-associated nephropathy. Patients should be screened at least annually by calculation of glomerular filtration rate and urinalysis for proteinuria. Black patients; those with CD4 cell counts of less than 200 per mm3 (0.20 × 109 per L) or viral loads of more than 4,000 copies per mL; and those with co-occurring diabetes, hypertension, or hepatitis C should be screened every six months.5,39 Patients with 1+ proteinuria or a glomerular filtration rate of less than 60 mL per minute per 173 m2 should have renal ultrasonography and 24-hour urine collection for protein analysis, and renal biopsy should be considered.39 Treatment includes angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, ART for patients with HIV-associated nephropathy, and steroids for adults with HIV-associated nephropathy.39 Protein restriction has not been proven beneficial.39
