
Am Fam Physician. 2011;84(8):883-886
Author disclosure: No relevant financial affiliations to disclose.
Lysteda is an oral antifibrinolytic licensed for the treatment of cyclic heavy menstrual bleeding (i.e., menorrhagia) of more than 80 mL per cycle. A competitive plasminogen inhibitor, it prevents plasmin formation and fibrinolysis of menstrual fluid. The extended-release formulation is designed to increase absorption time, thereby decreasing adverse gastrointestinal effects.
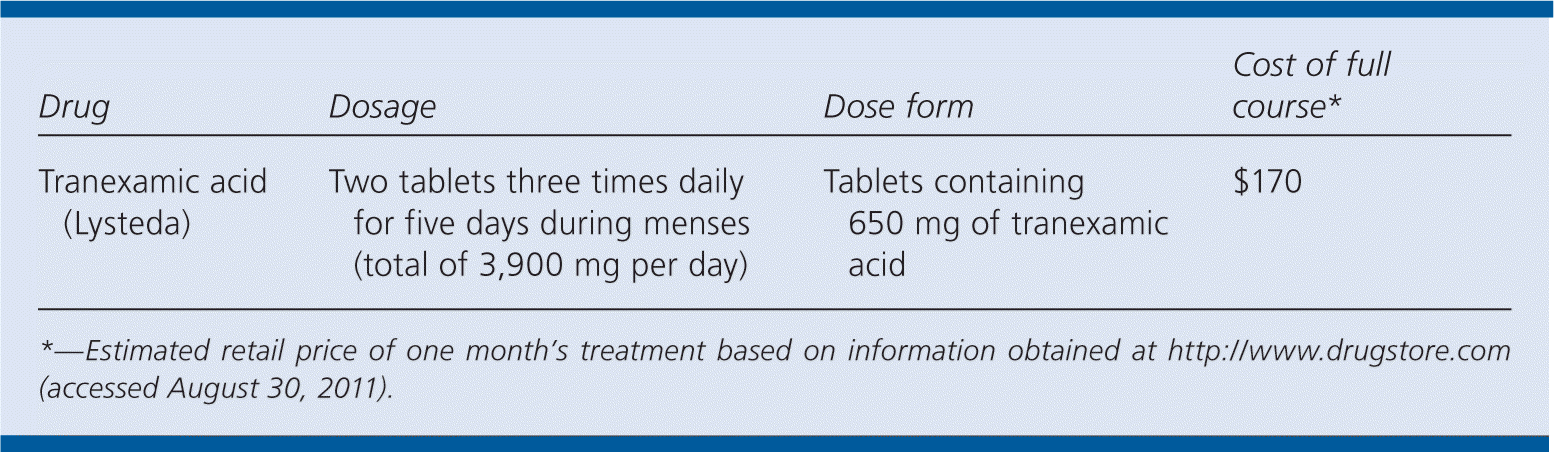
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Tranexamic acid (Lysteda) | Two tablets three times daily for five days during menses (total of 3,900 mg per day) | Tablets containing 650 mg of tranexamic acid | $170 |
SAFETY
Tranexamic acid theoretically increases the risk of venous thromboembolism (VTE). There have been case reports of venous and arterial thrombosis, including retinal occlusions, in patients receiving older formulations.1 However, there were no reports of VTE in studies of Lysteda2 or in most population studies of older formulations3; one case-control study showed a nonsignificant trend toward increased rates of VTE.4 Patients with a history of or predisposition to thrombosis should not take tranexamic acid. There are no data about the risk of VTE in patients receiving tranexamic acid with combined oral contraceptives; therefore, these drugs should not be used together unless there is a strong medical need. Tranexamic acid is a U.S. Food and Drug Administration pregnancy category B drug.
TOLERABILITY
Women taking tranexamic acid report menstrual cramps, headache, back pain, anemia, arthralgia, allergies, fatigue, or muscle cramps more often than those taking placebo.2 Withdrawals from clinical studies are similar among patients receiving tranexamic acid and those receiving placebo (24 and 26 percent, respectively).2
EFFECTIVENESS
Tranexamic acid reduces menstrual blood loss, but it does not affect the underlying cause of the dysfunctional uterine bleeding. In clinical studies of premenopausal women with cyclic heavy menstrual bleeding (40 percent of whom had uterine fibroids), tranexamic acid reduced the quantity of menstrual bleeding by 40 to 65 percent.1,2 This change was deemed meaningful by participants. In two randomized placebo-controlled studies, women who received 3,900 mg of tranexamic acid per day had a significant reduction in limitations on social, leisure, and physical activities.1,2 Tranexamic acid appears to improve quality of life by the third cycle in 80 percent of women.5 No studies have examined whether tranexamic acid prevents or treats iron deficiency anemia. Tranexamic acid has a beneficial effect in women for up to six cycles.1,2 No studies have compared the new formulation of tranexamic acid with other therapies. However, studies of variable design have compared older formulations with other agents. Studies of women with idiopathic or intrauterine device–related menorrhagia showed that tranexamic acid reduced average menstrual blood loss by 34 to 59 percent within three cycles,6–10 and was more effective than nonsteroidal anti-inflammatory drugs,6,9,10 oral luteal phase norethindrone,8 and cyclic medroxyprogesterone acetate (Provera).11 One study found tranexamic acid to be less effective than intrauterine administration of levonorgestrel (Mirena).9
PRICE
A single cycle of Lysteda costs approximately $170.12
SIMPLICITY
Bottom Line
Tranexamic acid is a nonhormonal option that reduces menstrual blood loss and improves quality of life in patients with heavy menstrual bleeding. It is more expensive than nonsteroidal anti-inflammatory drugs and hormonal therapies, but is an option when these treatments are not desired or recommended.
