
Am Fam Physician. 2011;84(12):1422-1425
| Guideline source: Centers for Disease Control and Prevention |
| Evidence rating system used? No |
| Literature search described? Yes |
| Guideline developed by participants without relevant financial ties to the industry? No |
| Published source:Morbidity and Mortality Weekly Report, July 8, 2011 |
| Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6026a3.htm |
Initiating appropriate contraception in the postpartum period is important to avoid negative outcomes related to short birth intervals. The postpartum period is an optimal time to initiate contraception because patients are often reassessing their health care and may be motivated to prevent another pregnancy.
The Centers for Disease Control and Prevention (CDC) recently reevaluated the safety of contraceptive use in the postpartum period. It subsequently updated its report, “U.S. Medical Eligibility Criteria for Contraceptive Use,” which was adapted from World Health Organization guidelines. Recommendations are categorized from 1 to 4 based on patient characteristics and the balance of benefits and harms of different contraceptive methods.
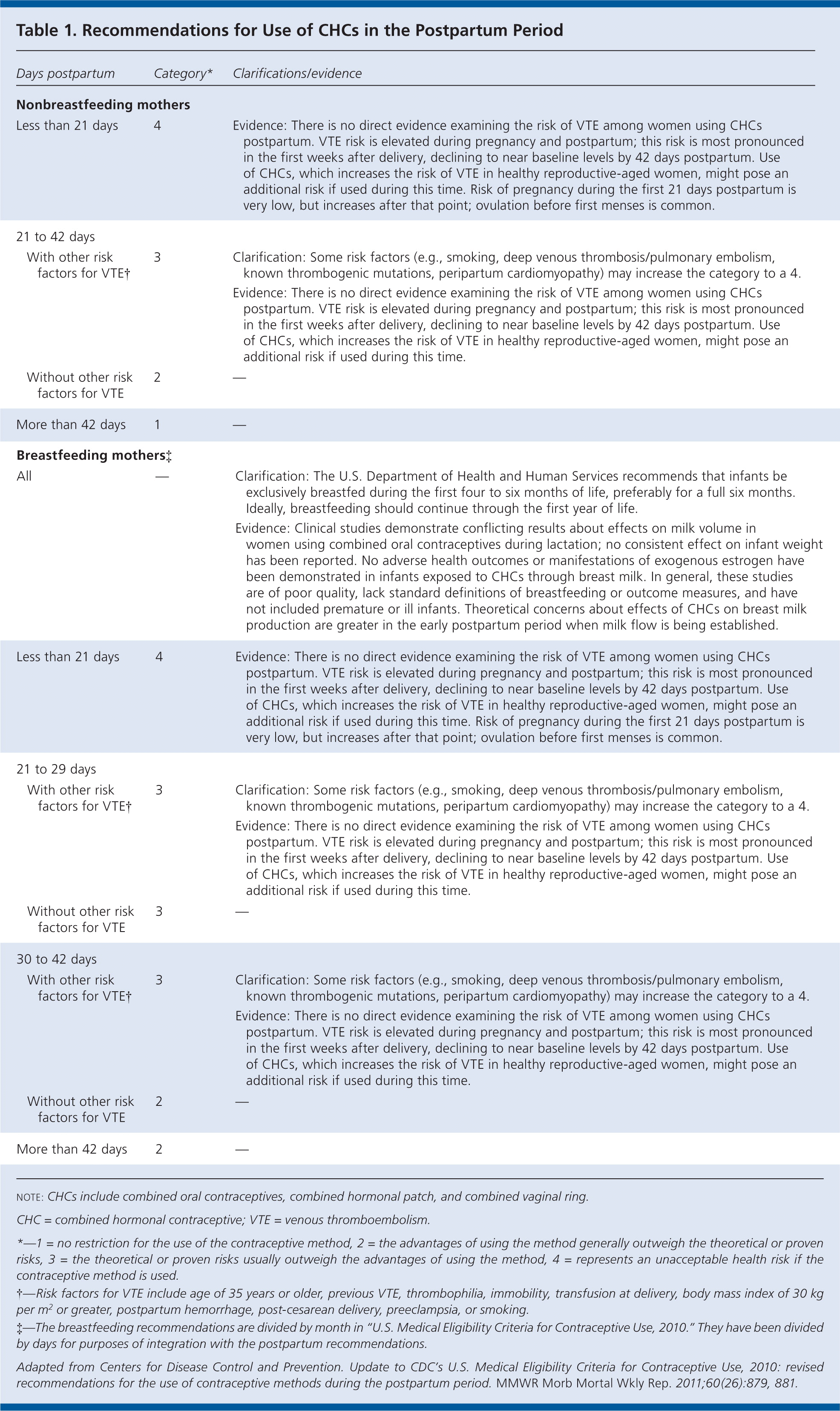
The CDC updated its recommendations to be more restrictive in the use of combined hormonal contraceptives during the first 42 days postpartum, especially in women with other risk factors for venous thromboembolism. Table 1 summarizes the new recommendations. In general, combined hormonal contraceptives should not be used during the first 21 days postpartum, and they should not be used up to 42 days postpartum in women with other risk factors for venous thromboembolism; they may be used without restriction after 42 days postpartum.
| Days postpartum | Category* | Clarifications/evidence | ||
|---|---|---|---|---|
| Nonbreastfeeding mothers | ||||
| Less than 21 days | 4 | Evidence: There is no direct evidence examining the risk of VTE among women using CHCs postpartum. VTE risk is elevated during pregnancy and postpartum; this risk is most pronounced in the first weeks after delivery, declining to near baseline levels by 42 days postpartum. Use of CHCs, which increases the risk of VTE in healthy reproductive-aged women, might pose an additional risk if used during this time. Risk of pregnancy during the first 21 days postpartum is very low, but increases after that point; ovulation before first menses is common. | ||
| 21 to 42 days | ||||
| With other risk factors for VTE † | 3 | Clarification: Some risk factors (e.g., smoking, deep venous thrombosis/pulmonary embolism, known thrombogenic mutations, peripartum cardiomyopathy) may increase the category to a 4. | ||
| Evidence: There is no direct evidence examining the risk of VTE among women using CHCs postpartum. VTE risk is elevated during pregnancy and postpartum; this risk is most pronounced in the first weeks after delivery, declining to near baseline levels by 42 days postpartum. Use of CHCs, which increases the risk of VTE in healthy reproductive-aged women, might pose an additional risk if used during this time. | ||||
| Without other risk factors for VTE | 2 | — | ||
| More than 42 days | 1 | — | ||
| Breastfeeding mothers‡ | ||||
| All | — | Clarification: The U.S. Department of Health and Human Services recommends that infants be exclusively breastfed during the first four to six months of life, preferably for a full six months.Ideally, breastfeeding should continue through the first year of life. | ||
| Evidence: Clinical studies demonstrate conflicting results about effects on milk volume in women using combined oral contraceptives during lactation; no consistent effect on infant weight has been reported. No adverse health outcomes or manifestations of exogenous estrogen have been demonstrated in infants exposed to CHCs through breast milk. In general, these studies are of poor quality, lack standard definitions of breastfeeding or outcome measures, and have not included premature or ill infants. Theoretical concerns about effects of CHCs on breast milk production are greater in the early postpartum period when milk flow is being established. | ||||
| Less than 21 days | 4 | Evidence: There is no direct evidence examining the risk of VTE among women using CHCs postpartum. VTE risk is elevated during pregnancy and postpartum; this risk is most pronounced in the first weeks after delivery, declining to near baseline levels by 42 days postpartum. Use of CHCs, which increases the risk of VTE in healthy reproductive-aged women, might pose an additional risk if used during this time. Risk of pregnancy during the first 21 days postpartum is very low, but increases after that point; ovulation before first menses is common. | ||
| 21 to 29 days | ||||
| With other risk factors for VTE † | 3 | Clarification: Some risk factors (e.g., smoking, deep venous thrombosis/pulmonary embolism, known thrombogenic mutations, peripartum cardiomyopathy) may increase the category to a 4. | ||
| Evidence: There is no direct evidence examining the risk of VTE among women using CHCs postpartum. VTE risk is elevated during pregnancy and postpartum; this risk is most pronounced in the first weeks after delivery, declining to near baseline levels by 42 days postpartum. Use of CHCs, which increases the risk of VTE in healthy reproductive-aged women, might pose an additional risk if used during this time. | ||||
| Without other risk factors for VTE† | 3 | — | ||
| 30 to 42 days | ||||
| With other risk factors for VTE † | 3 | Clarification: Some risk factors (e.g., smoking, deep venous thrombosis/pulmonary embolism, known thrombogenic mutations, peripartum cardiomyopathy) may increase the category to a 4. | ||
| Evidence: There is no direct evidence examining the risk of VTE among women using CHCs postpartum. VTE risk is elevated during pregnancy and postpartum; this risk is most pronounced in the first weeks after delivery, declining to near baseline levels by 42 days postpartum. Use of CHCs, which increases the risk of VTE in healthy reproductive-aged women, might pose an additional risk if used during this time. | ||||
| Without other risk factors for VTE † | 2 | — | ||
| More than 42 days | 2 | — | ||
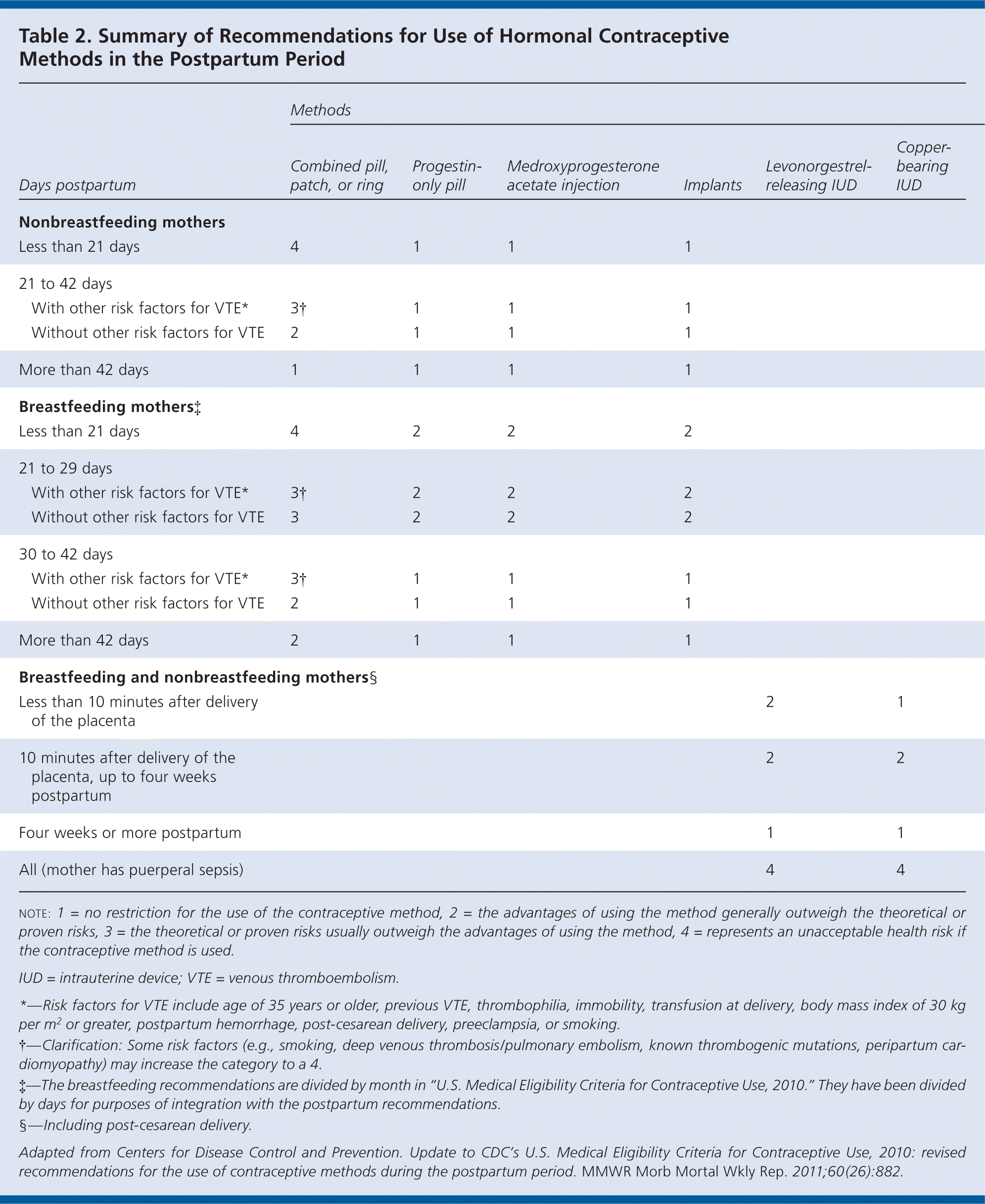
The CDC did not change its recommendations for the use of other contraceptive methods during the postpartum period (Table 2). Progestin-only hormonal contraceptives and intrauterine devices are safe for breastfeeding and nonbreastfeeding women and can be initiated immediately postpartum.
| Days postpartum | Methods | ||||||
|---|---|---|---|---|---|---|---|
| Combined pill, patch, or ring | Progestin-only pill | Medroxyprogesterone acetate injection | Implants | Levonorgestrel-releasing IUD | Copper-bearing IUD | ||
| Nonbreastfeeding mothers | |||||||
| Less than 21 days | 4 | 1 | 1 | 1 | |||
| 21 to 42 days | |||||||
| With other risk factors for VTE* | 3† | 1 | 1 | 1 | |||
| Without other risk factors for VTE | 2 | 1 | 1 | 1 | |||
| More than 42 days | 1 | 1 | 1 | 1 | |||
| Breastfeeding mothers‡ | |||||||
| Less than 21 days | 4 | 2 | 2 | 2 | |||
| 21 to 29 days | |||||||
| With other risk factors for VTE* | 3† | 2 | 2 | 2 | |||
| Without other risk factors for VTE * | 3 † | 2 | 2 | 2 | |||
| 30 to 42 days | |||||||
| With other risk factors for VTE* | 3† | 1 | 1 | 1 | |||
| Without other risk factors for VTE | 2 | 1 | 1 | 1 | |||
| More than 42 days | 2 | 1 | 1 | 1 | |||
| Breastfeeding and nonbreastfeeding mothers§ | |||||||
| Less than 10 minutes after delivery of the placenta | 2 | 1 | |||||
| 10 minutes after delivery of the placenta, up to four weeks postpartum | 2 | 2 | |||||
| Four weeks or more postpartum | 1 | 1 | |||||
| All (mother has puerperal sepsis) | 4 | 4 | |||||
