
Am Fam Physician. 2012;85(6):577-586
Author disclosure: No relevant financial affiliations to disclose.
Purpose
In AFP Journal Club, three presenters review an interesting journal article in a conversational manner. These articles involve “hot topics” that affect family physicians or “bust” commonly held medical myths. The presenters give their opinions about the clinical value of the individual study discussed. The opinions reflect the views of the presenters, not those of AFP or the AAFP.
Article
Patel MR, Mahaffey KW, Garg J, et al.; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
How does rivaroxaban (Xarelto) compare with warfarin (Coumadin) for stroke prevention in patients with nonvalvular atrial fibrillation?
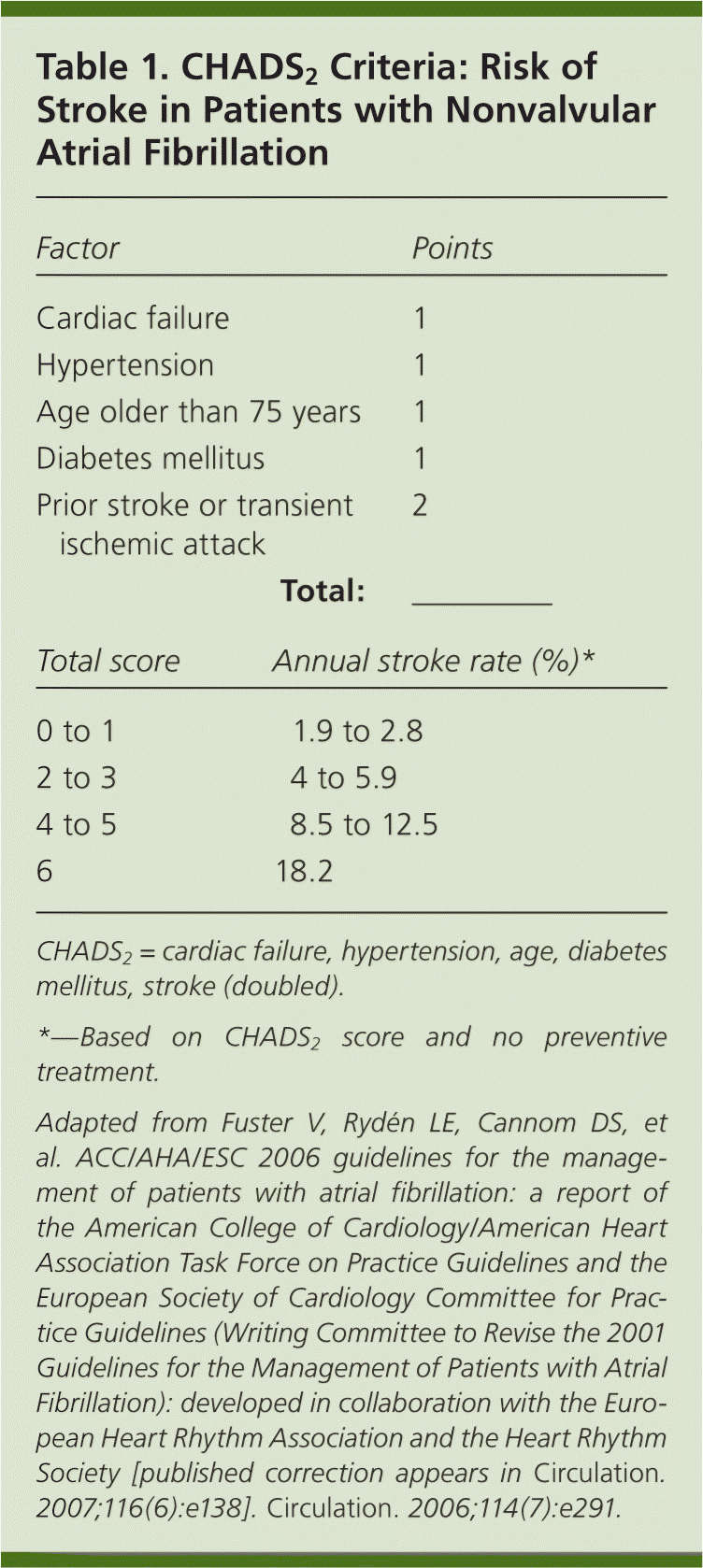
Andrea: Atrial fibrillation occurs with increasing prevalence as the patient population ages. The overall prevalence is estimated at 0.4 to 1 percent, with lower rates in patients younger than 60 years. In patients older than 80 years, the prevalence of atrial fibrillation increases to 8 percent and the annual risk of stroke ranges from 3 to 8 percent. Indeed, more than one-third of all strokes in octogenarians occur in those with atrial fibrillation. Since the early 1990s, oral vitamin K antagonists have been the mainstay of primary and secondary stroke prevention in patients with atrial fibrillation, particularly in those with moderate to high risk of stroke as defined by the CHADS2 (cardiac failure, hypertension, age, diabetes mellitus, stroke [doubled]) score (Table 11). However, these agents are limited by the need to monitor for appropriate therapeutic effect (international normalized ratio [INR] goal of 2.0 to 3.0), frequent dosage changes, an increased risk of bleeding, and multiple drug interactions.1 An ideal alternative medication for these highly effective agents would have similar preventive benefits, little or no monitoring requirements, and few drug interactions.
| Factor | Points | |
|---|---|---|
| Cardiac failure | 1 | |
| Hypertension | 1 | |
| Age older than 75 years | 1 | |
| Diabetes mellitus | 1 | |
| Prior stroke or transient ischemic attack | 2 | |
| Total:_________ | ||
What does this article say?
Andrea: The authors of this study randomized 14,264 patients to receive adjusted-dose warfarin (target INR of 2.0 to 3.0) or the direct factor Xa inhibitor rivaroxaban (20 mg daily or 15 mg daily if creatinine clearance was 30 to 49 mL per minute per 1.73 m2 [0.50 to 0.82 mL per second per m2]). Patients in both groups also received placebo pills to maintain blinding (those in the rivaroxaban group who received warfarin placebo also had sham INR results and adjustments). This randomized, double-blind, double-dummy trial was conducted at 1,178 participating sites in 45 countries. Patients were enrolled if they had atrial fibrillation and were at moderate to high risk of stroke based on a CHADS2 score of 2 points or more. More than one-half of the patients in each arm of the trial had experienced a previous stroke, embolism, or transient ischemic attack. The study was designed to determine if rivaroxaban is noninferior to warfarin in the prevention of stroke or systemic embolism. Additionally, the authors evaluated the safety of rivaroxaban with respect to major and nonmajor bleeding episodes.
The authors used a per-protocol analysis and determined that the number of primary events (a composite of stroke and systemic embolism) in each group was equivalent, suggesting that rivaroxaban is noninferior to warfarin. Specifically, 188 patients (1.7 percent per year) in the rivaroxaban group had a stroke or systemic embolism compared with 241 patients (2.2 percent per year) in the warfarin group (hazard ratio [HR] = 0.79; 95% confidence interval [CI], 0.66 to 0.96; P < .0001 for noninferiority). The composite safety end point of major and nonmajor clinical bleeding events was similar between the two groups with 14.9 and 14.5 percent per year, respectively (HR for rivaroxaban = 1.03; 95% CI, 0.96 to 1.11). Intracranial hemorrhage was less common in the rivaroxaban group with 0.5 percent per year versus warfarin with 0.7 percent per year (HR = 0.67; 95% CI, 0.47 to 0.93; P = .02); gastrointestinal bleeding was more common in the rivaroxaban group with 3.2 percent versus warfarin with 2.2 percent (P < .001). Based on this information, the authors concluded that rivaroxaban is noninferior to warfarin in the prevention of stroke and embolic events in patients with nonvalvular atrial fibrillation. The U.S. Food and Drug Administration approved rivaroxaban for stroke prevention in patients with atrial fibrillation on November 4, 2011.2
Should we believe this study?
Andrea: Noninferiority trials are becoming more common in the medical literature as a way to compare “new” and “old” treatment options in various disease processes without having to give patients placebo. In the case of the drugs evaluated in this study, it would be unethical to treat patients with CHADS2 scores of 2 points or greater with placebo because we know that warfarin can effectively reduce the risk of stroke in these patients. Noninferiority trials may be reasonable if the comparator drug could provide benefit with respect to lower cost, fewer adverse effects, or ease of administration compared with the standard treatment.
By definition, a noninferiority trial is designed to show that an alternative intervention is not unacceptably worse than the standard intervention. These trials do not have to meet the rigorous design and statistical format of more traditional superiority trials, nor are they designed to show superiority to the standard treatment. Noninferiority trials can be challenging to accurately design and interpret. To combat these issues, the CONSORT (Consolidated Standards of Reporting Trials) group established a checklist for appropriate reporting of noninferiority trials in 2006.3 The authors of this study were able to meet these criteria. Unfortunately, the flaws inherent in even well-designed noninferiority trials make it difficult to believe the claims in this study.
Noninferiority trials must declare a margin of how far outside the acceptable outcome their drug can perform and still be considered noninferior to the standard treatment.4 In this study, the authors used a noninferiority margin (similar to relative risk) of 1.46 as the prespecified target. Put another way, they intended to interpret a 46 percent clinical difference in the rate of stroke or systemic embolism between rivaroxaban and warfarin as clinically nonsignificant. This margin of difference is much greater than I would be willing to accept for my patients when considering such a life-altering event as stroke or embolism.
Bob: Additionally, P values reported in noninferiority studies merely indicate that the HR is statistically different from the declared margin, not that the drugs being evaluated are statistically different from each other. So, the impressive P values in this study merely show that the difference in the effect of the drugs was statistically better than the prespecified margin, not that rivaroxaban is superior to warfarin.
Mark: A main tenet of noninferiority trials is that the efficacy of the standard treatment (in this case, warfarin) remains preserved when compared with trials that established its efficacy. A large analysis of the literature on warfarin has shown that patients in a variety of settings remained in therapeutic range (INR of 2.0 to 3.0) an average of at least 63 percent of the time.5 Given that the time in therapeutic range is directly related to risk of stroke and thromboembolic events, it is imperative that patients in the warfarin group of any noninferiority study achieve the same time in therapeutic range. The patients taking warfarin in this trial were in therapeutic range only 55 percent of the time (quartiles ranged from 43 to 71 percent of the time), which may have reduced warfarin's overall effectiveness in stroke and embolism prevention. This would make rivaroxaban appear better than it really is.
Bob: Noninferiority trials are often assessed using per-protocol, rather than intention-to-treat, approaches. In theory, this is because intention-to-treat analyses are more likely to demonstrate no difference between drugs because of patient dropout and missing data. It is important to note that per-protocol analysis is also subject to similar bias, particularly in large studies with multiple sites, such as this trial with 1,178 participating sites in 45 countries. The authors reported that one site was excluded from analysis because of protocol violations, and another had questionable data quality but the patients were still included in analysis. Additionally, there was regional variation in time to therapeutic dose of warfarin and no protocol for standard warfarin dosing. It is hard to believe that the study protocol was strictly followed at the 1,176 other sites.
What should the family physician do?
Andrea: There are too many variables in this study that allow rivaroxaban to appear comparable to warfarin for me to be comfortable using this as a first-line agent for stroke prevention in my practice. I'm especially wary of transitioning existing patients whose conditions are well-controlled (i.e., INR easily maintained in therapeutic range) to rivaroxaban, because it isn't clear that the warfarin arm of this study included patients who had sufficient time in therapeutic range.
Bob: Keep in mind that there is also a cost issue—a 30-day supply of rivaroxaban costs $262 (compared with $6.65 for a 30-day supply of warfarin, 5 mg).6 The other new oral anticoagulant, dabigatran (Pradaxa), is also expensive. Many insurance carriers are not covering the cost of these new agents.
However, I understand two of the downsides of warfarin: (1) patient frustration with the travel and time associated with frequent INR testing and (2) inconsistency in staying within the therapeutic range. One way around this dilemma is home self-monitoring and self-management. A recent Cochrane review of 18 randomized trials (4,723 participants) revealed a statistically significant decrease in thromboembolic events, hemorrhage, and all-cause mortality in patients capable of self-monitoring at home.7
Mark: From a pharmacology standpoint, it should be noted that rivaroxaban has no reversal agent and many drug interactions, most notably cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ketoconazole, itraconazole [Sporanox], clarithromycin [Biaxin]) and P-glycoprotein inducers (e.g., rifampin, carbamazepine [Tegretol], phenytoin [Dilantin], St. John's wort).6 So for now, I'll be waiting for a superiority comparison of warfarin and rivaroxaban before I can feel justified in making this transition. However, now that rivaroxaban is approved by the U.S. Food and Drug Administration for stroke prevention in patients with nonvalvular atrial fibrillation, it's unlikely that the drug manufacturer will sponsor this much more rigorous type of trial.
Main Points
Patients with nonvalvular atrial fibrillation and a CHADS2 score of 2 points or more should be placed on warfarin anticoagulation. If they do not meet the CHADS2 criteria for warfarin, then they should receive therapy with aspirin.
If a patient's condition is well-controlled on warfarin, this study does not support transitioning him or her to rivaroxaban, the more expensive alternative.
Home monitoring of INR should be considered for patients who are capable and motivated to perform self-monitoring.
Rivaroxaban has no reversal agent and has significant drug interactions (P-glycoprotein inducers and CYP3A4 inhibitors increase the risk of bleeding; P-glycoprotein inducers reduce effectiveness).
EBM Points
Noninferiority trials are designed to show that an alternative treatment is not substantially worse than the standard intervention. They do not meet the same rigorous design and statistical format of traditional superiority trials.
Authors of noninferiority trials must declare a margin of how far outside the acceptable outcome the study drug can perform and still be considered noninferior to the standard treatment. In this study, the authors determined that a margin of 46 percent difference was within the acceptable range for warfarin and rivaroxaban in the prevention of stroke or embolism.
The efficacy of the standard treatment (in this case, warfarin) shown in the trials that established its efficacy must be preserved in any noninferiority trials. Time in therapeutic range was not within established norms for many of the patients in this study—this will make warfarin perform worse and allow rivaroxaban to appear noninferior.
